2025 AIChE Annual Meeting
(231f) Selective Crystallization: Development of a Seeding Strategy to Purify Lysozyme in an Impurity-Containing Batch Process
During the design and operation of the protein crystallization process, it is crucial to ensure critical quality attributes (CQAs) meet desired specifications. A key challenge encountered during the design process is the presence of foreign substances/impurities in the solution which could inhibit crystallization due to a variety of possible reasons. Some impurities may prevent the clustering of solute molecules, whether physically blocking the nuclei from clustering together to form a crystal lattice, or chemically by forming complexes. Impurities could also embed themselves into the lattice structure, reducing product purity. Aside from this, impurities may increase/decrease solubility of the material in the solution. In the former, lower supersaturations could prevent crystal formation altogether, and in the latter, nucleation could occur too quickly due to higher supersaturations resulting in poor control. Therefore, understanding the effect of impurities on crystallization and ways to counteract them is crucial for successful process design.
In this work, we present an investigation into the effect of adding impurities to a batch precipitant-induced lysozyme crystallization process, and the development of a seeding protocol for selective crystallization in two parts: 1) Initially obtaining system understanding from unseeded lysozyme experiments (NaCl as precipitant) followed by the addition of impurities such as thaumatin, albumin as well as other proteins and amino acids; and 2) Using existing seeding protocols from related work, observing and theorising the effect of silica seeds on impurity rejection and selective crystallization.
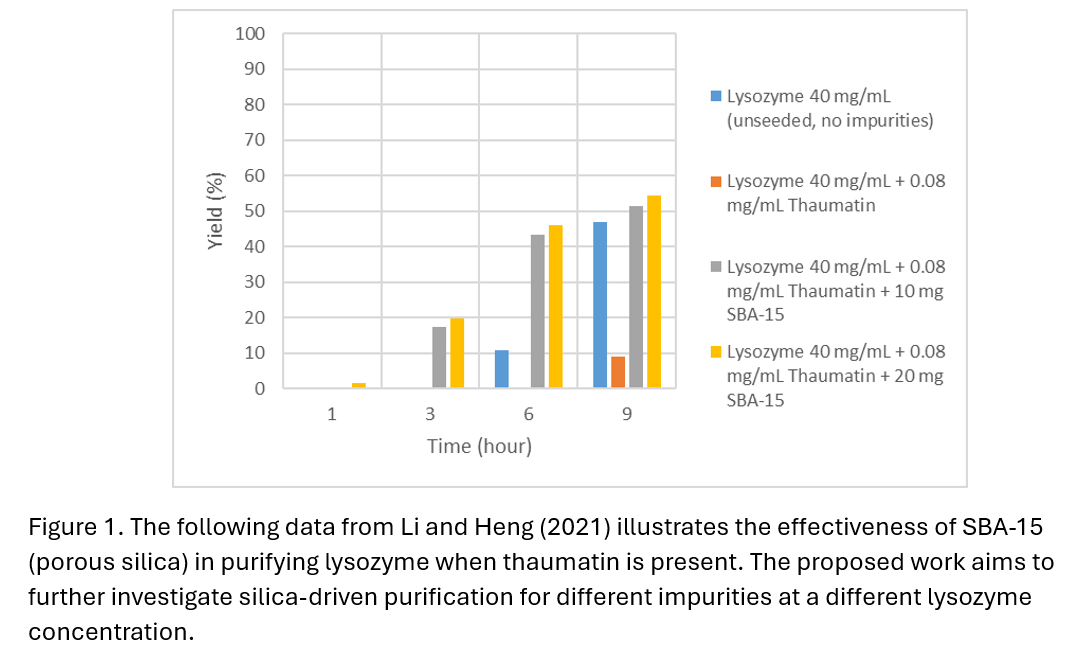
Initially, 50 mL unseeded lysozyme crystallization experiments at room temperature were first conducted at varying supersaturations. These were guided by existing solubility data from literature, with the objective of obtaining an unseeded induction time of around 100-200 minutes to ensure seed-enhanced nucleation does not cause the induction time to be reduced too significantly, and the duration of experimental runs is feasible. Building on published work by Li and Heng (2021), Li et al. (2020), and Chen et al. (2021), impurities such as thaumatin, albumin, other proteins and amino acids were added to the system to obtain experimental data for comparison. As the impurities had a large inhibitory effect on lysozyme crystallization, silica seeds of varying porosity (nonporous and mesoporous), pore sizes (~4nm) and particle sizes (<150 μm) were added were added to impurity-containing solutions to induce crystallization, with the effect on crystal purity and induction time investigated. To monitor lysozyme concentration in the solution, UV-Vis spectrophotometry measurements (Thermo Scientific NanoDrop One) were taken at regular intervals.
It was found that in some cases such as for thaumatin and albumin, silica seeding proved effective in separating lysozyme from an impurity-containing solution by inducing heterogeneous nucleation. Increased impurities required more silica to overcome its effects on the system.
In summary, this work aims to provide an initial foray into the effect of impurities on protein crystallization, and the potential of applying seeds such as silica to allow selective crystallization. The implication of this work is that seeding could be explored as a way to solve impurity problems when crystallising from protein synthesis crudes, and if successful could accelerate the adoption of protein crystallisation as a go to purification method in industry.
References:
Li X. and Heng, J. Y. Y. (2021) Protein crystallisation facilitated by silica particles to compensate for the adverse impact from protein impurities. CrystEngComm, 23 (47), 8386-8391. DOI: 10.1039/d1ce00983d
Li, X., Chen, W., Yang, H., Yang, Z. and Heng, J. Y. Y. (2020) Protein crystal occurrence domains in selective protein crystallisation for bio-separation. CrystEngComm, 22(27), 4566-4572. DOI: 10.1039/d0ce00642d
Chen W., Cheng, T. N.H., Khaw L. F., Li X., Yang H., Ouyang J., Heng, J. Y. Y. (2021) Protein purification with nanoparticle-enhanced crystallisation. Separation and Purification Technology, 255, 117384. DOI: 10.1016/j.seppur.2020.117384.