2025 AIChE Annual Meeting
(524e) Selective Catalytic Dehydrogenation of Liquid Organic Hydrogen Carriers through Visible Light Photolysis
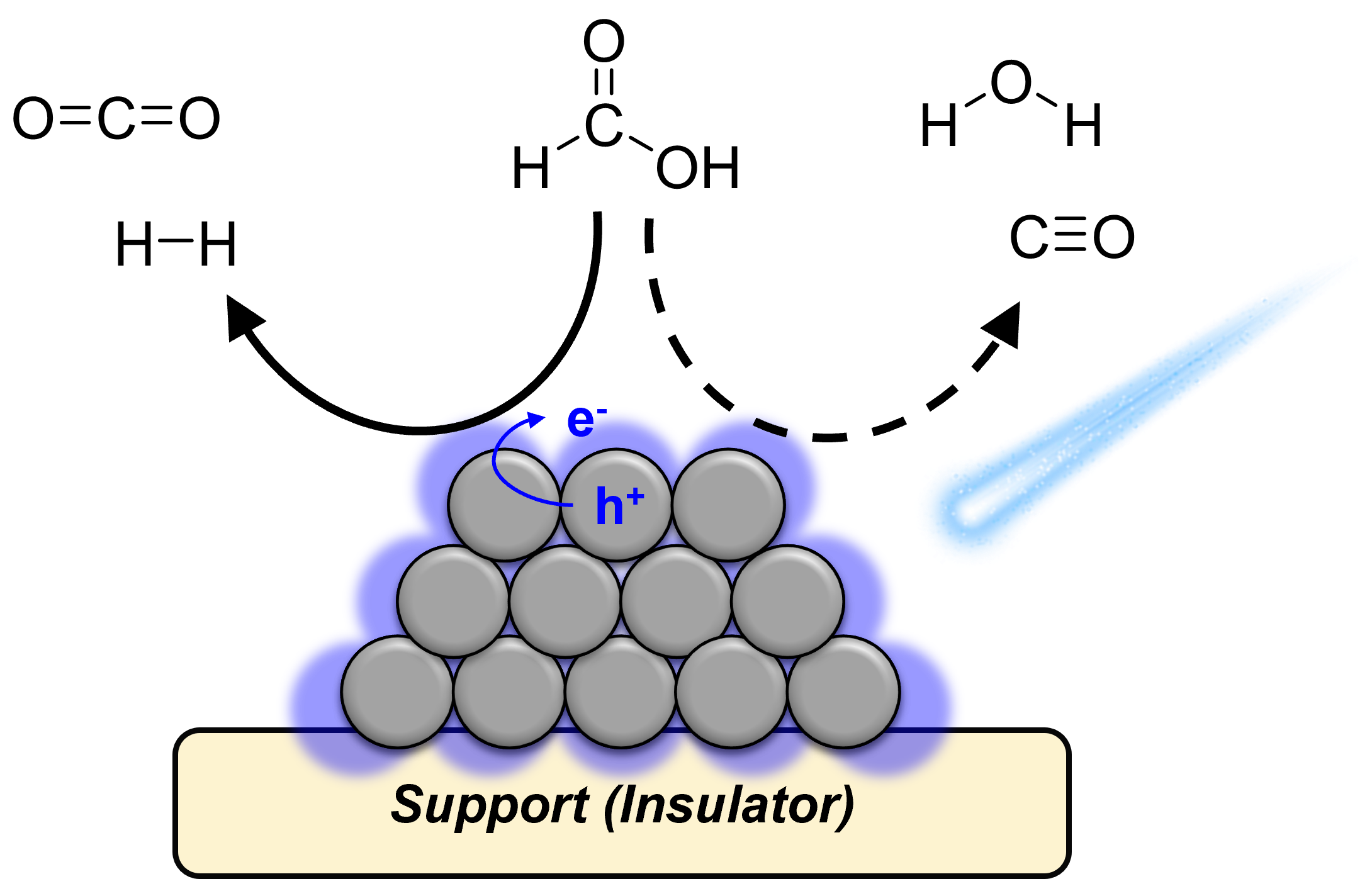
The dehydrogenation of liquid organic hydrogen carriers (LOHC) on catalytic surfaces face significant kinetic barriers, exacerbated by the accumulation of surface spectator species, limiting their utility for on-demand hydrogen delivery. While thermo-, electro-, and photo-catalytic approaches have been demonstrated for the selective dehydrogenation of liquid organic hydrogen carriers, the cooperative use of multiple energetic stimuli remains under-utilized. Using precious metal catalytic surfaces that facilitate the thermal dehydrogenation of formic acid, we investigated the ability of visible light to selectively promote the rate of catalytic turnover through electronic excitation of kinetically relevant intermediates. Over Pt nanoparticles supported on insulating metal oxides (e.g. silica, alumina), a continuous wave of 440 nm light significantly accelerated the overall catalytic rate of formic acid decomposition. The rate enhancement was selective towards the desired dehydrogenation, with the rate of turnover being more than 95% selective to formic acid dehydrogenation over dehydration. Regardless of temperature, an apparent quantum yield of ~ 30 % was observed to the desired dehydrogenation of formic acid. The rate of formic acid photolysis was found to be insensitive to temperature, resulting in a negligible apparent activation energy (Ea,app ~ 0 kJ/mol) consistent with a non-thermal catalytic process. The difference in the rate of dehydrogenation over dark (thermal only) and illuminated surfaces demonstrates the selective acceleration of chemical catalysis through photolysis, by targeting kinetically relevant intermediates that are responsive to light.