2025 AIChE Annual Meeting
(437e) Scaling Down Fluid Bed Drying: A Comparative Study of Mini-Glatt and Consigma™-1
Authors
Therefore, the primary objective of this study was to evaluate whether the Mini-Glatt fluid bed dryer could serve as a viable downscale model for the drying process in CG-25. This was attained by comparing Mini-Glatt drying performance with that of ConsiGma-1 (CG-1), a smaller-scale unit within the same system of CG-25. A Design of Experiments (DoE) was conducted, in both Mini-Glatt and CG-1, incorporating the same five tests with airflow rates ranging from 70 to 90 m³/h and drying temperatures set at 40, 50, and 60°C.
Furthermore, an FBD model to guide scale-up was developed and two calibration approaches compared. First, the adequate gas flow rate is computed from a fluidization regime map with minimum velocity for fluidization, entrainment velocity and fluidization regime using correlations generally available. The drying step is predicted using a model similar to the one described in (Gavi et al., 2020), where dimensionless drying rate curve, mass transfer coefficient, and desorption curve were calibrated solely from dynamic vapor sorption data. In the second approach, the gas flow rate is predicted from pressure drop vs gas velocity data retrieved from Mini-Glatt. Similarly, the drying kinetics component of the FBD model is calibrated from experiments conducted in Mini-Glatt.
The starting material consisted of wet granules composed of microcrystalline cellulose (MCC) and lactose in identical proportions, and PVP K30 as a binder, with a liquid-to-solid (L/S) ratio of 33%. The granules used in CG-1 fluid-bed drying experiments were produced in CG-1 itself, whereas the granules used in Mini-Glatt were produced in the Pharma 11 extruder, which has a considerably smaller screw diameter, that directly impacts the final granule size. Granules produced on the larger scale are significantly larger due to the larger gap size in the granulator, as well as screw diameter, however, these differences are probably eliminated after milling (Franke et al., 2023).This phenomenon was observed in the conducted granulation experiments, as CG-1 granules evidenced an increased PSD due to the larger screw diameter compared to Pharma 11 extruder.
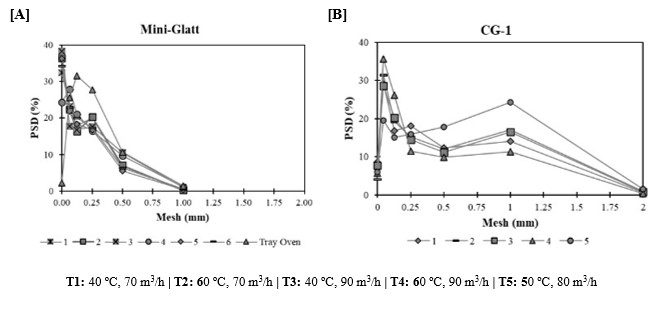
After the drying step, a sieve shaker analysis was performed to assess the final PSD of the granules. In the Mini-Glatt drying trials, a control sample dried in a tray oven was used for comparison. As expected, the control sample exhibited larger granules since, unlike fluid bed drying, the tray oven process does not expose the material to particle collisions, contact with equipment walls, or drying gas convection forces. While Mini-Glatt trials showed a higher fraction of fines, the PSD curves remained consistent across different drying conditions, indicating that drying parameters did not influence granule breakage. This observation aligns with findings from previous studies (Vandevivere et al., 2022). Regarding CG-1 PSD results, a considerable proportion of granules exceeded 0.5 mm in size. However, the fine fraction was comparable to that observed in Mini-Glatt (~38%), reinforcing the similarity in drying outcomes between the two systems. Furthermore, the PSD curves across all trials were closely aligned, supporting that drying parameters did not impact granule breakage, regardless of the equipment used.
These findings provide valuable insights for process scale-up and optimization in continuous pharmaceutical manufacturing. They highlight the feasibility of using Mini-Glatt as a predictive downscale model for ConsiGma-25 drying operations, supporting reproducibility and product quality.