2025 AIChE Annual Meeting
(693c) Scalable Pore Incorporation of Single-Layer Graphene Membranes for Carbon Capture
Authors
Jian Hao - Presenter, EPFL
Kumar Varoon Agrawal, École Polytechnique Fédérale De Lausanne (EPFL)
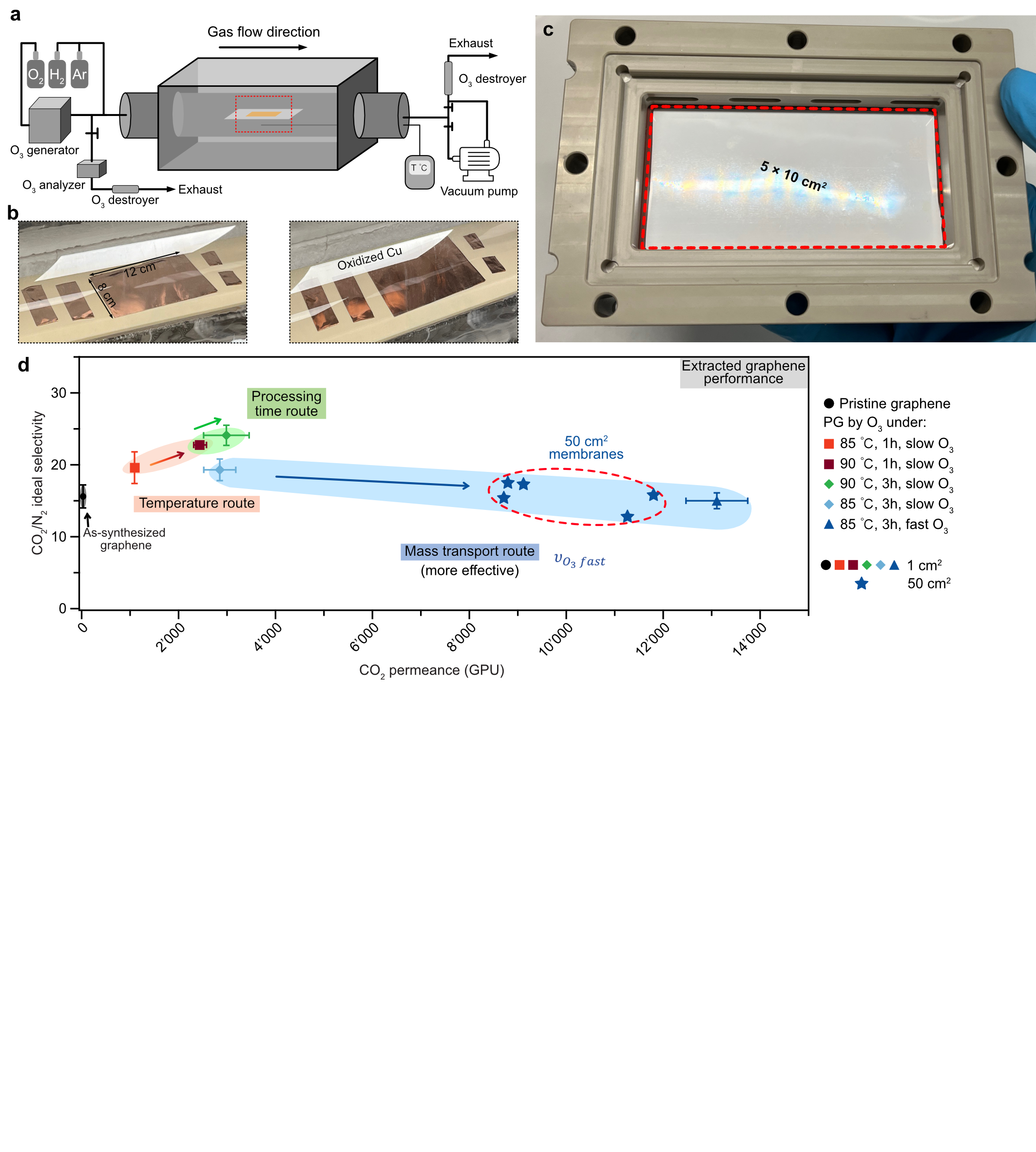
Nanoporous atomically thin membranes (NATM) have emerged as a promising platform for effectively separating molecules where the size difference is extremely close, e.g., separating CO2 and N2 for carbon capture.1 However, this field generally faces an outstanding challenge in the scalability of NATM. Single-layer porous graphene (PG) membranes, one of the most popular and well-studied NATM, have shown a competitive capture penalty owing to their low gas permeation resistance compared to polymeric membranes.2 Here, we present several advances toward scaling up PG membranes for carbon capture. We demonstrate that high-quality single-layer graphene can be synthesized on industrial-scale, low-cost Cu foils, and large-area (50 cm2) PG membranes can be prepared with a user-friendly transfer protocol. 3,4 This proves that graphene membranes can be scaled up, with potentially reduced cost. However, incorporating gas-sieving pores in graphene over a large area remains an unsolved challenge. Pores in graphene are carbon vacancy defects and can be formed by removing atoms from the basal plane. Prior works have shown that exposing graphene under ozone is an effective approach to form pores.5 However, they involve sophisticated setups that are challenging to scale up. In this work, a large reactor for pore incorporation was implemented (Figure a), which allowed the incorporation of uniform pores in large-area graphene in a reproducible manner (Figure b). PG membranes were successfully prepared with an area up to 50 cm2 (Figure c). A systematic study of graphene oxidation revealed that O3 mass transfer (velocity), as opposed to the commonly studied reaction kinetics (temperature and time), dominates pore formation kinetics (Figure d). The methods discussed here will form the basis of roll-to-roll production of graphene membranes with attractive performance in the important application of carbon capture.
Figure a) A schematic illustration of the large-scale ozone functionalization setup. b) Photos of graphene resting on Cu before and after oxidation. c). Photo of successfully prepared 5 × 10 cm2 graphene membrane. d) Gas permeation results of as-synthesized graphene (black) and PG (colored) membranes at 1 cm2 and 50 cm2 scale. The ozone oxidation was optimized by different reaction routes: temperature, processing time, and mass transport routes. The permeance of PG is extracted from the membrane using the resistance model.
References:
- Villalobos, L. F., Babu, D. J., Hsu, K.-J., Van Goethem, C. & Agrawal, K. V. Gas Separation Membranes with Atom-Thick Nanopores: The Potential of Nanoporous Single-Layer Graphene. Acc. Mater. Res. (2022) doi:10.1021/accountsmr.2c00143.
- Micari, M., Dakhchoune, M. & Agrawal, K. V. Techno-economic assessment of postcombustion carbon capture using high-performance nanoporous single-layer graphene membranes. J. Membr. Sci. 624, 119103 (2021).
- Hao, J. et al. Scalable synthesis of CO2-selective porous single-layer graphene membranes. (in press) Nat. Chem. Eng. (2025) doi:10.1038/s44286-025-00203-z.
- Hao, J. et al. Scalable synthesis of CO2-selective porous single-layer graphene membranes. Preprint at https://doi.org/10.26434/chemrxiv-2025-z8g2d (2025).
- Huang, S. et al. In situ nucleation-decoupled and site-specific incorporation of Å-scale pores in graphene via epoxidation. Adv. Mater. 34, 2206627 (2022).