2025 AIChE Annual Meeting
(333c) Salt Ions Accumulation in Bipolar Membranes Limits the Maximum Rate of Neutralisation
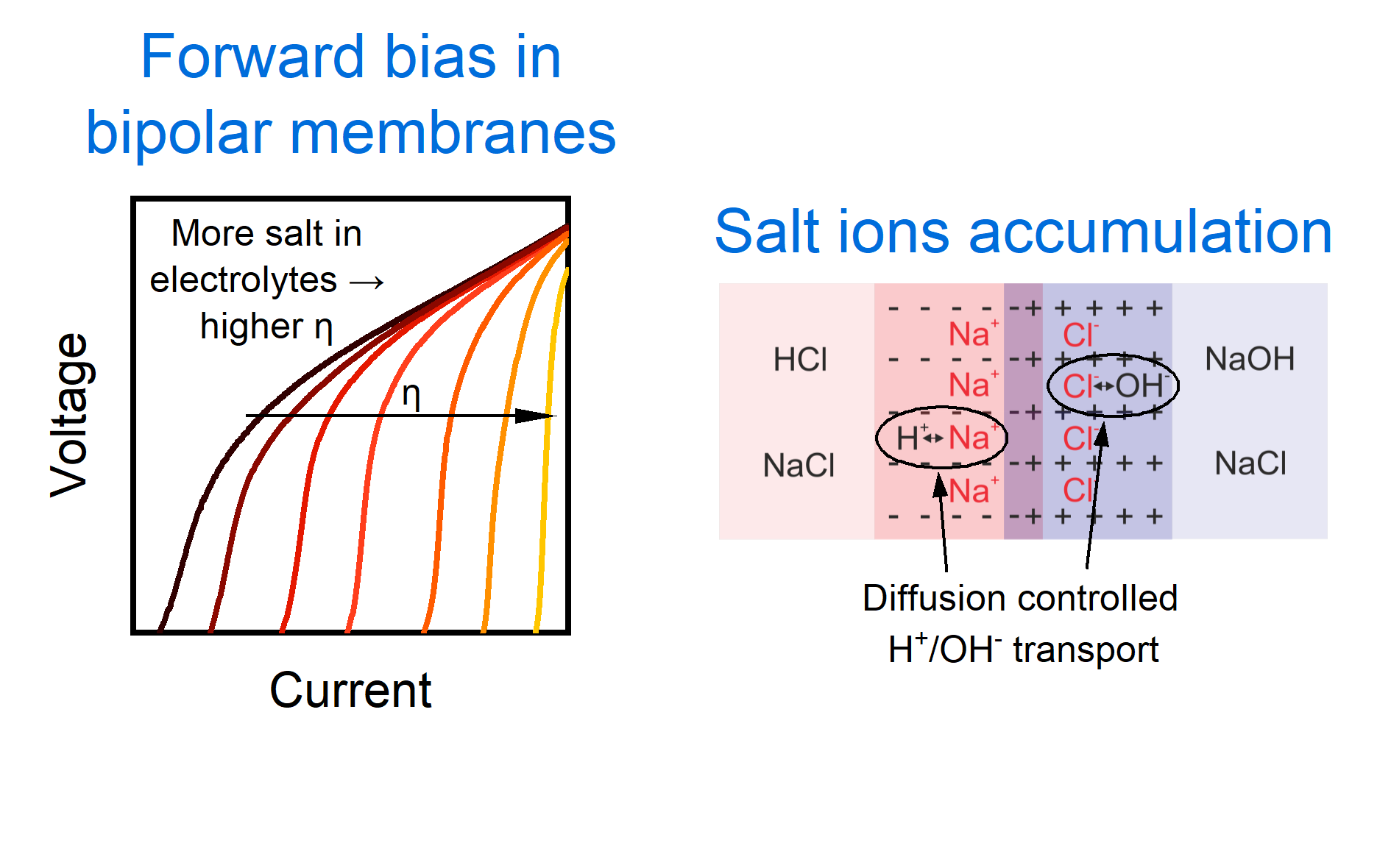
In recent years, BPMs operating under forward bias have garnered particular attention due to their potential to enable energy recovery from pH gradients and facilitate ion-selective electrochemical conversion. However, their performance in real systems is often lower than expected. One of the main challenges arises from the contamination of acid and base electrolytes with salt ions – such as Na+ and Cl- - which typically occurs due to ion crossover in closed-loop systems or during long-term operation.
While previous studies have shown that the presence of salt can degrade BPM performance, the underlying mechanism has remained unclear. Specifically, the question of how salt ions influence H+ and OH- transport during forward bias operation has not been adequately addressed. In this study, we investigate the origin of salt-induced limitations in BPMs under forward bias, focusing on the emergence of ionic blockade and how it governs the maximum neutralisation rate. Using electrochemical impedance spectroscopy (EIS) alongside polarisation measurements in a controlled cell setup, we identify the key transport phenomena responsible and propose strategies for mitigation.