2025 AIChE Annual Meeting
(429i) Role of Linkage Chemsitry, Side Chain Polarity, and Side Chain Length on the Ion Aggregation and Conductivity in Polymerized Ionic Liquids
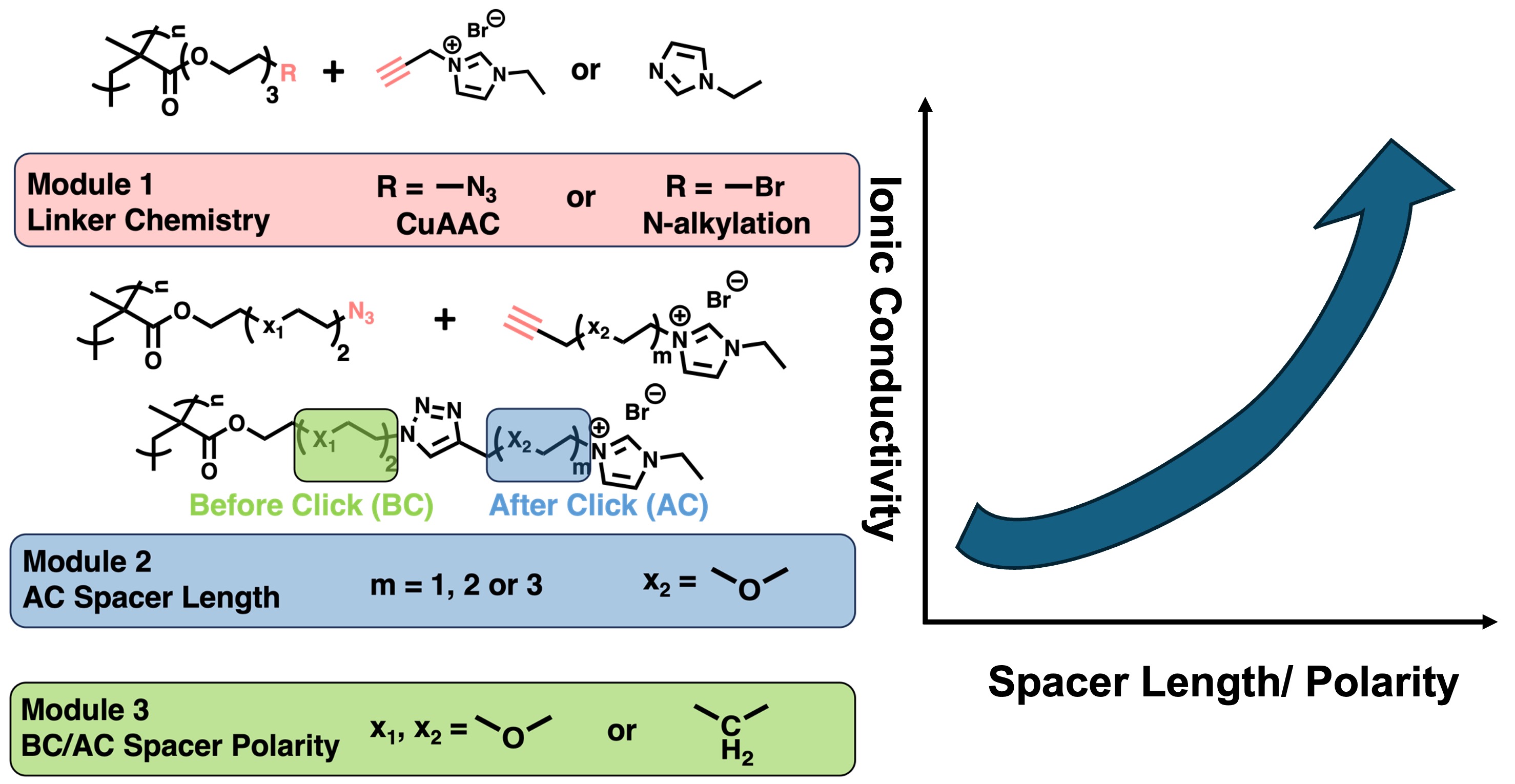
Investigating the relationship between structure and properties is fundamental in the realms of science and engineering. For solid polymer electrolytes, the correlation between chemical composition and ion transport is typically elucidated by synthesizing diverse monomers followed by polymerization which is usually synthetic heavy and has less precise control over the molecular weight. In this study, various solid-state polyelectrolytes were synthesized by employing a post-polymerization functionalization approach via Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC). This offers an efficient strategy to delineate the structure-property relationship. Firstly, the role of linkage chemistry on the structure and ion transport was examined. Then, utilizing a methacrylate backbone with an azide as clickable group at chain end, the effects from both different side chain lengths ranging from 19 to 28 atoms, and different local polarity of the side chain were also systematically investigated. We found that (1) the triazole ring structure generated from click chemistry negatively impact the ionic conductivity (2) the longest side chain closer to the ionic group result in greater than 10-fold increase in ionic conductivity at 30 °C comparing to the shortest side chain, (3) the higher ether oxygen content shows 5-fold increase in ionic conductivity at 30 °C comparing to only alkyl side chains while the position of the ether oxygen plays a less significant role in ionic conductivity. These results illustrate the role of both side chain length and polarity plays an important role in determining the ion transport of polymerized ionic liquids. Further, this work demonstrates the post-polymerization method is a versatile way to understand the structure-property relationship in solid-state polyelectrolytes and have the potential to be explored as a tool for high-throughput experimentations.