2025 AIChE Annual Meeting
(180at) The Role of Cations in Water Freezing: An in-Situ Raman Study
Authors
Aakash Kumar, Stony Brook University
Krishnakumari Pamula, Stony Brook University
Amol Pophali, Stony Brook University
Dilip Gersappe, Stony Brook University
Tae Jin Kim, Stony Brook University
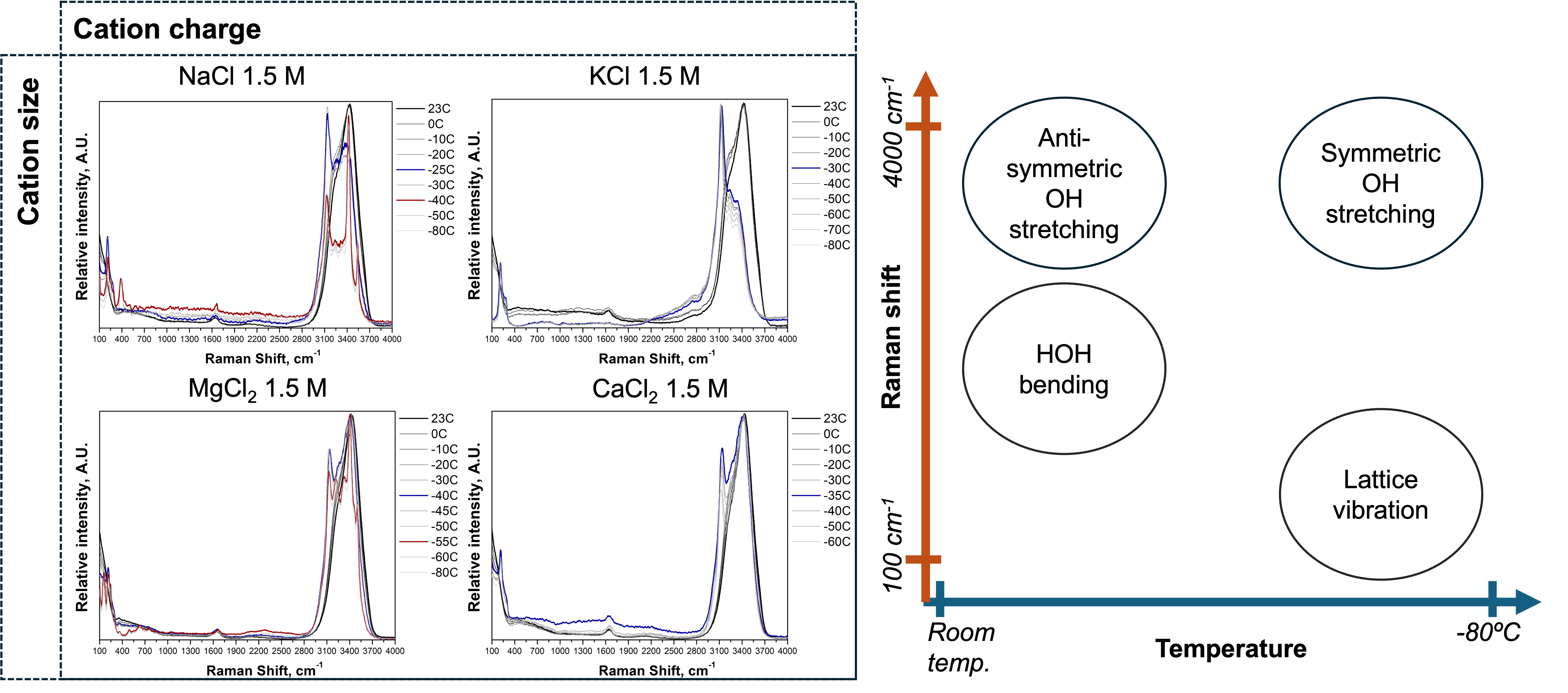
Salts are widely used as effective anti-freezing agents in the development of advanced materials such as anti-icing coatings, freeze-resistant hydrogels, and low-temperature electrolytes1,2. Understanding how salts influence water’s freezing behavior is essential for optimizing the performance and reliability of such materials under subzero conditions. Raman spectroscopy is a powerful tool for probing molecular structure; however, in-situ Raman studies on the freezing behavior of aqueous salt solutions remain limited. In this study, in-situ Raman spectroscopy was employed to investigate water’s freezing behavior in the presence of chloride-based salts, highlighting cation-specific effects associated with variations in ionic charge and size. Raman spectra were collected as the temperature was gradually reduced from room temperature to -80ºC. The results show that salts with higher ionic charges and stronger hydration effects significantly disrupt tetrahedral water networks, leading to lower phase change temperatures. Distinct phase behaviors, including the formation of salt crystals or hydrates, were identified through Raman peak changes in the crystal lattice vibration and OH stretching regions. Additionally, mixed-phase systems were observed at lower temperatures due to localized salt enrichment in the remaining liquid phase. These findings provide molecular-level insights into salt-water interactions and offer design principles for next-generation anti-freezing materials.
- Jian, Y. et al. Biomimetic anti-freezing polymeric hydrogels: keeping soft-wet materials active in cold environments. Materials Horizons 8, 351–369 (2021).
- Zhang, X.-F. et al. Inorganic Salts Induce Thermally Reversible and Anti-Freezing Cellulose Hydrogels. Angewandte Chemie International Edition 58, 7366–7370 (2019).