2025 AIChE Annual Meeting
(18g) The Role of Arteriovenous Graft Curvature in Haemodynamics: An Image-Based Approach
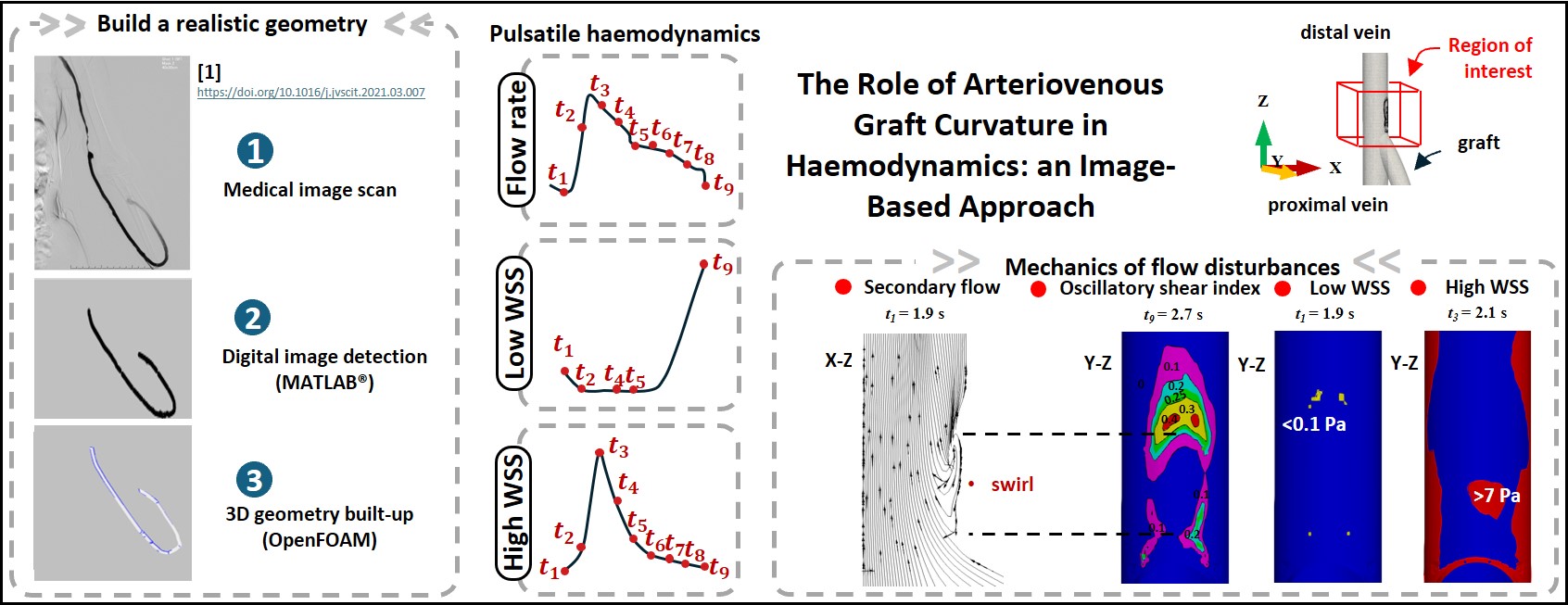
This work firstly introduces an image-based approach to support CAD-based simulations. Using adjustable fibres, we created in vitro AVG models in both loop and straight fashions, with one end of the graft stiched to the artery and the other end to the vein. These models exhibited a broad variety of vein-to-graft anastomotic angles between 10° and 75° whilst artery-to-graft anastomosis remained constant. The fibres representing the graft were controllable and all had a fixed length of 310 mm. To digitize the physical models, we captured them using a smartphone, followed by image processing through a custom MATLAB algorithm. The detected vessel skeletons, therefore, provided the ‘path’ for constructing the three-dimensional geometry in OpenFOAM. This simple, yet accurate, method allowed us to improve the accuracy of geometries, thus facilitating comparisons between the primary variable of interest, i.e. vein-to-graft anastomotic angles.
The artery, graft and vein typically had respective lumen dimeters of 4 mm, 6 mm and 8 mm, and herein lies a potential impact factor. To address the anatomical mismatch between the graft and the artery, where the graft generally has a larger diameter, two arterial-end graft modifications were examined in this work: (1) a short (10 mm) tapered conical segment and (2) an enlarged intersection using a free flap. Both simulated a surgeon’s technique to support decision-making in surgical practice.
The free, open-source tool box OpenFOAM was selected to map the haemodynamics. Blood flow simulations were treated as a transient flow of a viscous, incompressible fluid. Given the vessel size was apparently larger than blood cells (~ 10-3 cm) and shear rate was large to neglect blood rheology, the fluid was assumed to be Newtonian, and therefore with a constant dynamic viscosity of 0.005 Pa•s and a constant density of 1060 kg/m3. The finite volume pisoFoam solver in OpenFOAM 10 was subsequently selected to solve the Navier-Stokes equations, which is a transient solver including time derivatives to account for the changes in velocity and pressure over time. A piecewise function for blood flow rate was coded to mimic the physiologically relevant conditions over a heart beating cycle. Mesh convergence and computational time studies were conducted to ensure solution accuracy.
The flow field and the evolution of the wall shear stress (WSS) over time were analysed to monitor the negative pulsing effect on the vessel wall patches, particularly at the vein-to-graft anastomosis. The results show that WSS values fluctuated in response to the pulsatile nature of blood flow in the brachial artery. A positive correlation was observed between the WSS values and the blood flow waveforms. The vessel wall encountered high percentage of pathological patches when the blood flow rate was high. These problematic regions, however, greatly fade away at the trough of low blood flow rate. In more detail, among the different configurations small vein-to-graft anastomosis led to reduced pathological regions under the effects of high WSS, i.e. vein-graft anastomosis at 13.2°.
To explore in-depth correlations, key metrics were defined to quantify the WSS that deviate from the normal physiological ranges of 0.1 to 7 Pa in the vein segment. Nine successive time points ( t0 = 1.8 s to t9 = 2.7 s) were monitored in a complete aortic cycle. The results show an initial decrease of low WSS arising from the acceleration of blood flow in the early phase of a circulatory loop. Since blood flow fell in the second phase, there was a remarkable increase of low WSS at the final time point, associated with the lowest blood flow rate measurement. The time-varying risk metrics differed by the order of magnitude of 0 to 2 on the dependence of the graft geometry and vein-to-graft anastomosis angles. The peak of high WSS occurred at t3 = 2.1 s in association with the highest blood flow measurement among the nine time points.
In the figure attached, one can see a swirl downstream of the blood flow at the vein-to-graft anastomosis where the two blood streams met. The curvature of the flow bend was determined by tracing blood streams originating from the graft. At t1 = 1.9 s, the curvature of the bend was estimated to be 45.6, yielding a Dean number of De = 99. At t3 = 2.1 s, the blood flow rate increased, and the curvature decreased to about 25 due to the changing flow path. This increased the Dean number significantly to De ~ 210, which resulted in unstable secondary flows and corresponding high WSS metrics. The secondary flow also means slow moving blood near the inner side of the anastomosis, that is, the blood flow rate near the inner wall was smaller than that in the outer side. Herein, low WSS was observed in the preceding and receding regions of the Dean swirl downstream of the anastomosis. This is in line with the clinical discoveries: the neoinitimal hyperplasia often occurs downstream of the graft-vessel anastomosis. When the arterial blood flow dropped off to the lowest point, these problematic patches spread to the bottom wall, and surprisingly, over an increased distal distance.
Pathological flow fields were mostly distributed downstream of the vein-to-graft anastomosis over a limited distance within 50 mm. Notably, high oscillatory flow (OSI > 0.25) was observed in the inner bend channel, in close proximity to the Dean vortex, and extended further into the downstream region. Regions of high WSS were primarily located preceding the Dean vortex and in the side wall patches of the bend. The Dean vortex enlarged at larger vein-to-graft anastomotic angles for both looped and straight graft types due to a reduced radius of curvature. In general, looped graft generated larger swirls because of higher velocity scales and greater graft inflow access. When the vein-to-graft anastomotic angle was below 20°, straight grafts exhibited predominantly unidirectional flow throughout the entire cardiac cycle. Looped grafts, however, continued to develop secondary flows but at higher arterial blood flow rates due to their greater graft inflow access. To our knowledge, no studies in arteriovenous haemodynamics have explicitly examined the impact of curved bends or the potential correlations between failure metrics and such geometric features.
To account for the accumulation process when fibrin built up at slow moving blood field, an integral of low WSS over all time points in the entire aortic cycle was summarised. Both looped and straight configurations demonstrate a decreasing trend with increased vein-to-graft anastomotic angles, however, straight grafts experienced more high risk regions impacted by low WSS. This is because straight grafts normally have reduced graft access due to high flow friction caused by the reversal of stream split the arterial end. The lower blood flow rate at vein-to-graft anastomosis likely accounts for the increased percentage of slow-moving blood in straight grafts.
The results show distinct responses to varying vein-graft anastomotic angles in terms of high WSS. Since high WSS causes endothelial cell dysfuncion, the strongest WSS values were used for subsequent configuration comparisons. Generally, both graft types showed a rising trend with larger vein-to-graft anastomotic angles which is in a good agreement with recent studies by Williams et al. and Pahlavanian et al. However, a notable difference in the magnitude is also evident. Looped grafts demonstrated a more pronounced sensitivity to these changes, resulting in significantly higher risk percentages. The risk curve for looped grafts reached a plateau at 60°, whereas the curve for straight grafts continued to rise gradually until it levelled off at 75°.
The oscillatory index numbers (OSI) were highly comparable for all graft types, particularly at large anastomotic angles (45° to 75°). Below 45° anastomotic angle, looped grafts were detected with more high-risk regions impacted by highly oscillatory flow patterns.
Interestingly, all the three risk metrics in tapered grafts show a consistent pattern to those in the looped configurations. This is not beyond our expectation given they both were configured in a looped manner. Owing to the reduction in graft access in tapered grafts, high WSS and high OSI fields were reduced whilst low WSS regions were enhanced. This suggests that the overall graft curvature has a major impact on the trend of the risk measurements, but the magnitude of the risk rates is on the dependence of graft access.
As a comparison, idealised models were built up separately for benchmark studies. Results from the idealised models show a reasonable agreement with the realistic models, albeit with some deviations. Their curve slopes become notably steeper. Moreover, the results demonstrate little dependence on the graft length. This is because fluid velocity profiles reach a fully developed regime after a certain distance in a straight pipe, the so-called entrance length.
Overall, this work presented an image-based approach that allows transformation of medical images into three-dimensional digital geometries. The use of hand-made models characteristically exemplifies real surgical applications on the one hand and effectively eliminates the side effects of irrelevant variables on the other. This study highlights that haemodynamics in arteriovenous grafts is significantly influenced by the global graft curvature (straight grafts vs. looped grafts) (idealised models vs. realistic models), vein-graft anastomotic angles and graft modifications at the arterial end (tapered vs. non-tapered). The findings suggest that looped graft configurations with moderate vein-graft anastomotic angles (30°-45°) are most effective in minimizing aberrant flow patterns, and therefore prolong overall graft survival rates. The results from this work have the potential to inform medical professionals about graft areas that are subject to high shear stresses due to the oscillating nature of blood flow as well as the graft geometric configuration when performing surgery. The model developed in this works allows personalization for individual patients’ needs and requirements.