2025 AIChE Annual Meeting
(320h) Revealing the Structural Dynamics of MoOx Catalysts through Static and Transient Raman Spectroscopy
Authors
Dhanush Thirulogachandar - Presenter, Rutgers, The State University of New Jersey
Thu Nguyen, Rutgers, The State University of New Jersey
George Tsilomelekis, Rutgers University
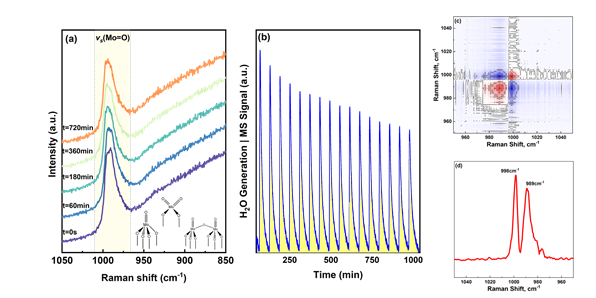
Supported molybdenum-based catalysts have gained interest for their efficiency in diverse chemical applications. Modifying the physicochemical characteristics of active molybdenum has proven to enhance its catalytic activity. However, the coexistence of multiple MoOx species introduces challenges in understanding the correlation between catalyst preparation and performance. Investigation on these materials have shown to have di-oxo structure and more complex structures in supports like SiO2, Al2O3 and TiO2 . This study presents a methodological approach for depositing (MoOx)n species on mixed CeO2-TiO2 and anatase TiO2 using the equilibrium deposition filtration (EDF) method. Surface species are analyzed through in-situ Raman spectroscopy with selective isotopic labeling under transient conditions. The work further examines the sequence and mechanism of oxygen site removal and substitution. The study employs EDF method to carefully impregnate the type of molybdenum species anchoring on the support and below the monolayer coverage. We then use advanced characterization like in-situ Raman coupled with isotopic labelling with 18O provides insights into the MoOx configurations. The results show that the distribution of the Molybdenum oxide depend mainly on the pH of the precursor solution in both the supports used. Analysis of the spectra shows that the structure of oxide to be mono-oxo. The framework is further extended by integrating pulse experiments with operando Raman spectroscopy to differentiate oxygen site reactivity. Combined results reveal that H2 exposure initially removes oxygen from terminal (Mo=O) sites, followed by disruption of some Mo–O–Support bonds. This enables oxygen exchange among various Mo–O bonds during reoxidation.