2025 AIChE Annual Meeting
(520c) Reactant-Dependent Stability of Supported Metal Catalysts for Hydrogen Storage in N-Heterocyclic Carriers
Authors
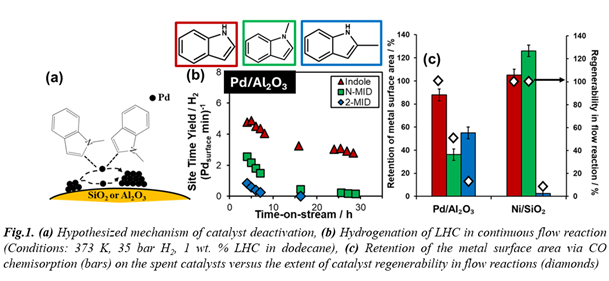
We synthesized 0.5 wt.% Pd- and Ni catalysts supported on SiO2 or Al2O3 by incipient wetness impregnation and evaluated their reactivity and stability for the hydrogenation of N-LHCs with varying methyl group positions. Continuous flow reactor studies assessed time-on-stream stability (Figure 1b) and regenerability (Figure 1c), while post-reaction characterization (CO chemisorption, microscopy) identified irreversible deactivation pathways. Both Pd and Ni underwent reversible deactivation due to coking, but sintering strongly depends on LHC structure and catalyst composition. The reactivity of Pd/SiO2 and Pd/Al2O3 was regenerated through calcination and reduction treatments following indole hydrogenation (achieving >5,900 catalytic turnovers, per surface metal site) but N- and 2-methylindoles caused severe catalyst sintering (Figure 1c). In contrast, Ni/SiO2 was regenerable for indole and N-methylindole hydrogenations but sintered during 2-methylindole hydrogenation (Figure 1c). Given that pyrrole rings are chelating ligands, we hypothesize that reaction-induced sintering occurs via chelation of metal adatoms (Figure 1a). We investigated additional LHCs with different basicities and steric properties, but no direct correlation between stability and basicity was observed, suggesting that steric and electronic factors both contribute to sintering. This work provides key insights that guide the design of robust catalysts to facilitate hydrogen storage in chemical bonds.