2025 AIChE Annual Meeting
Rationalizing Specific Ion Effects in Electrochemical Nitrate Reduction Using Machine Learning Potential Simulations
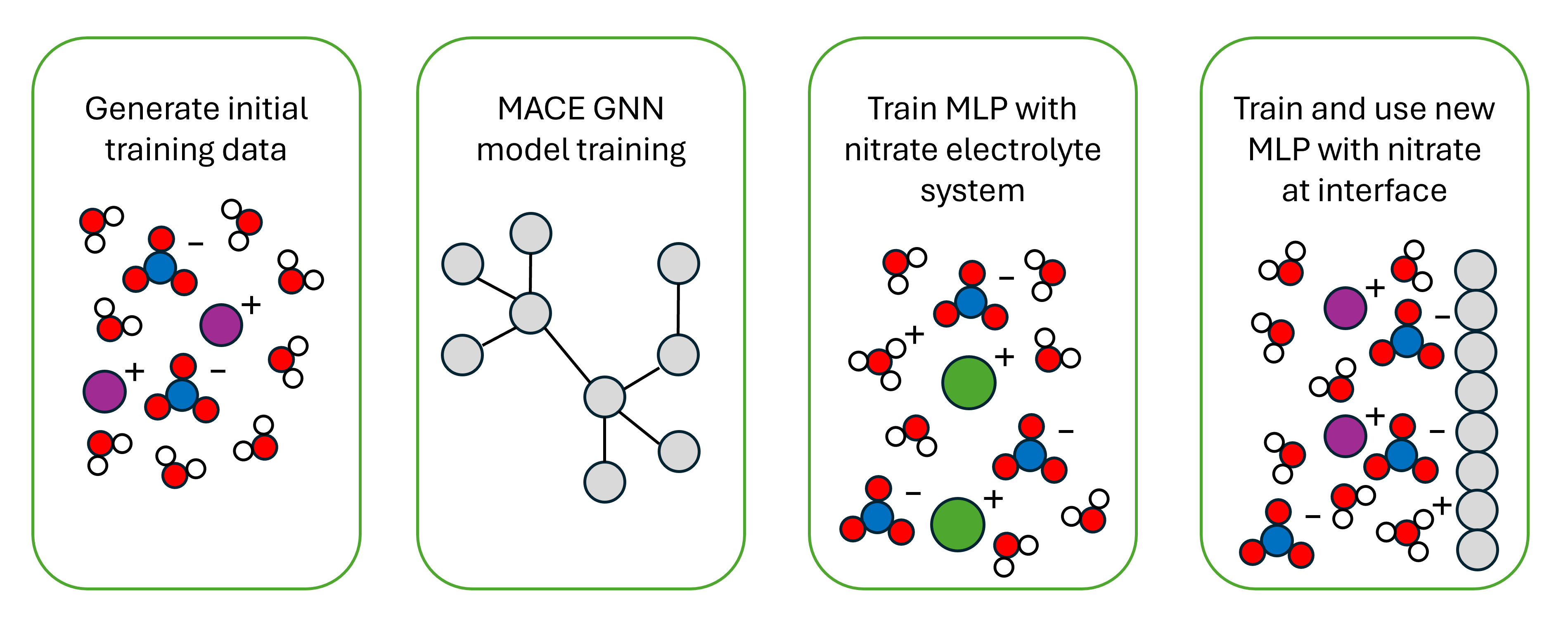
Ammonia serves as a key component in fertilizers that help address the issue of world hunger. However, the primary method of producing ammonia currently is through the Haber-Bosch process, which consumes elemental nitrogen and converts it to ammonia. Due to this process using elemental nitrogen as an input, ammonia and its subsequent reactive products, one of which being nitrate, build up in the environment from this anthropogenic increase with no sink. As such, these ammonia-based fertilizers have increased nitrate-based water pollution by disrupting the natural nitrogen cycle. Electrochemical nitrate reduction to ammonia presents a promising way to both produce ammonia and help close the nitrogen cycle by consuming nitrates. In this process, though, there are many aspects that are not well understood, one of which being the effect of pH and specific cations on nitrate density and distribution near the electrode. Understanding this would greatly aid the effort of developing more effective electrolytes for this process. One way to study this is through atomistic simulations, which allow detailed characterization and visualization of each interaction near the electrode electrolyte-metal interface. Unfortunately, in terms of atomistic simulation methods, studying this with density functional theory is very computationally expensive, to the point of not being able to run on long enough timescales and large enough simulation boxes. On the flip side, traditional molecular dynamics has accuracy limitations and restrictions, not being able to model proton donation or electrolyte-metal interfaces in general. A rapidly emerging method of solving this problem is the development of a machine learning potential. Machine learning potentials are trained off of accurate density functional theory calculations and learn to output forces and potentials that are close to density functional theory in accuracy for a much lower computational cost. As such, we trained a machine learning potential on the bulk electrolyte and soon the electrolyte-metal interface as well to bridge the gap of accuracy and cost to give an atomistic view and study the properties of nitrate near a TiH2 electrode interface, which has been experimentally shown to be an effective electrode for nitrate reduction.