2025 AIChE Annual Meeting
(7e) Rates and Regioselectivities of Epichlorohydrin Methanolysis Depend on the Topology of Aluminosilicate Catalysts
Authors
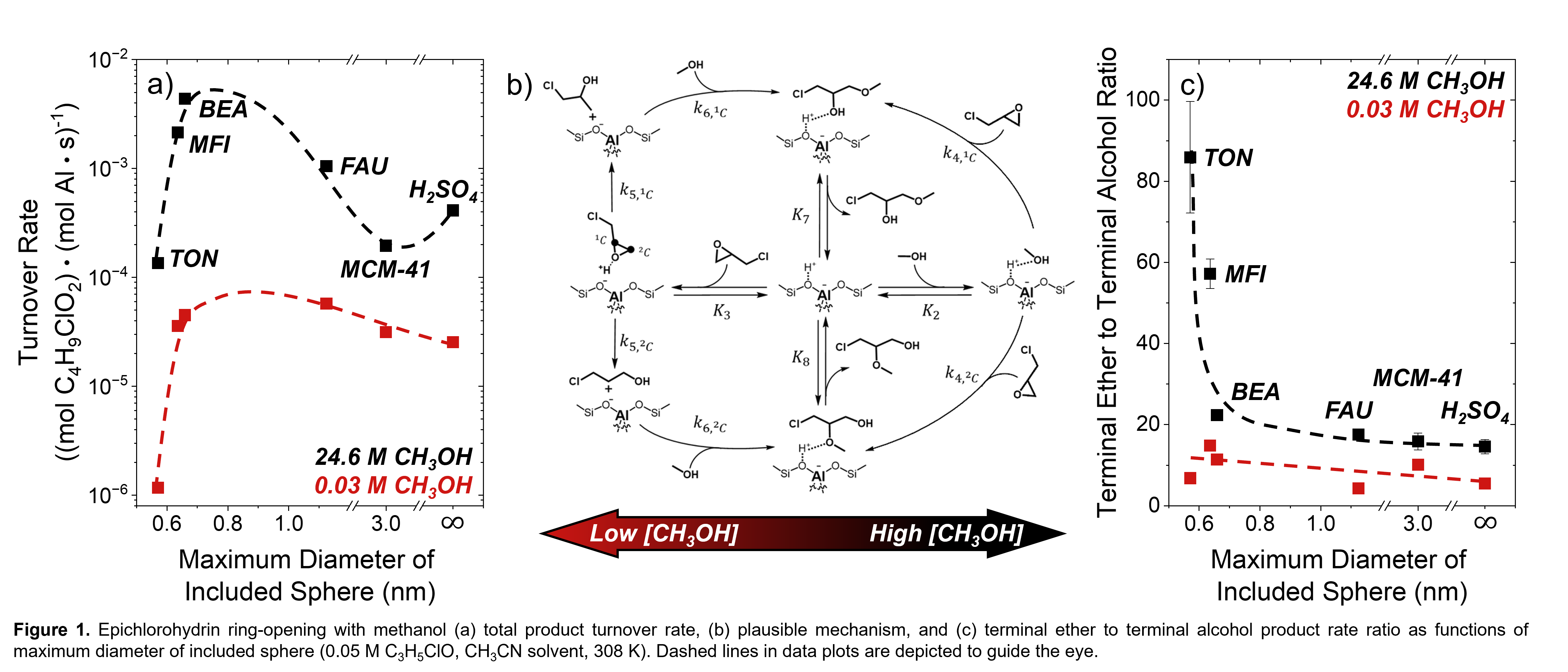
Here, we describe differences in rates and regioselectivities of epichlorohydrin ring-opening with methanol (CH3OH) across a series of Brønsted acidic aluminosilicates of distinct topologies and offer molecular interpretations of the solvating interactions that give rise to these effects. Turnover rates for ring-opening reactions increase at all methanol concentrations with increasing pore size from TON (0.57 nm) to BEA (0.66 nm) before decreasing for larger pore sizes (Figure 1a). The magnitude of rate decreases observed in larger pore materials depends on the kinetic regime (Figure 1b), with rates most sensitive to pore size at high [CH3OH] when bulky epichlorohydrin reacts in an SN2 pathway with a CH3OH-derived surface species. In this regime, apparent activation enthalpies and entropies change non-monotonically with pore size and reflect differences in transition state stabilities due to intrapore solvation of protonated intermediates. These effects selectively stabilize transition states for the sterically accessible terminal ether product as the pore size decreases from H2SO4 to TON (Figure 1c). Conversely, regioselectivities remain similar across the examined pore sizes at low [CH3OH] when an SN1 pathway involving epichlorohydrin-derived surface species dominates (Figure 1b), which reveals protic solvents confer greater kinetic sensitivity to material topology for liquid-phase zeolite catalysis.