2025 AIChE Annual Meeting
(363b) Rapid Modular Continuous Flow Platform Assembly for Process Chemistry Development.
Authors
In this work, we describe the development of a modular continuous flow platform to significantly expedite process chemistry development, enabling rapid and versatile reconfiguration for diverse reaction screening. The system seamlessly integrates various reactor types, including tubular plug flow reactors (PFR), plate reactors, and continuously stirred tank reactors (CSTR), alongside essential hardware components such as pumps, temperature control units, pressure sensors, and flow meters. A programmable logic controller (PLC) and a user-friendly, web-based human-machine interface (HMI) facilitate real-time monitoring and precise control of process parameters from any laboratory computer, while comprehensive data logging supports thorough post-experiment analysis and optimization.
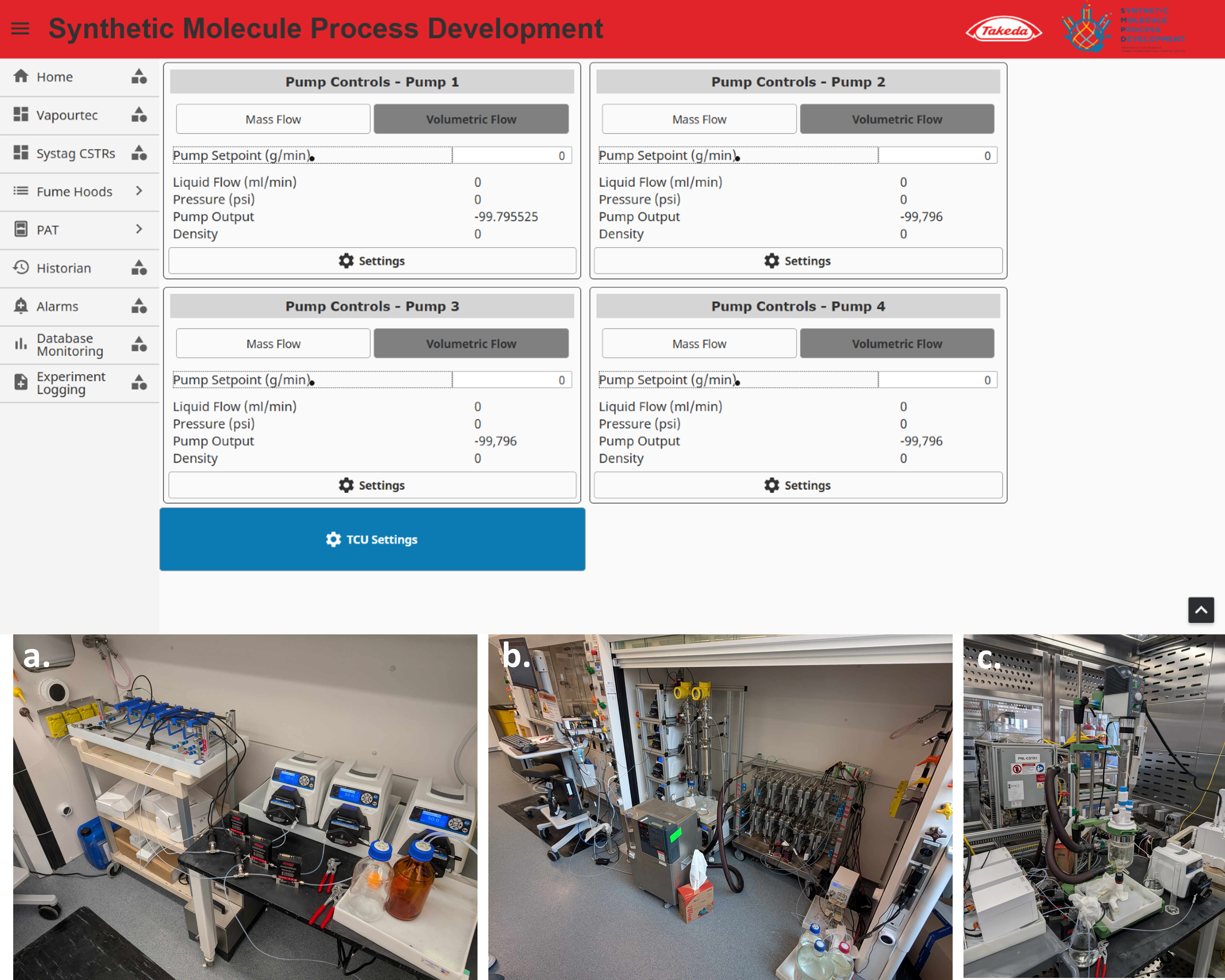
The platform's exceptional versatility and speed were rigorously demonstrated through a series of practical applications. Initially, a proof-of-concept (POC) study was successfully conducted using a Corning Low flow reactor (Figure 1a.) in a PFR configuration, validating the system's core functionality. Subsequently, the platform was rapidly reconfigured, within a matter of days, to incorporate a Corning G1 SiC reactor (Figure 1b.), achieving a substantial 500g production run within a mere two hours. This significant achievement showcased the system's efficient scale-up capabilities and its potential for rapid production. Leveraging the produced material, the platform was further reconfigured into a CSTR (Figure 1c.) within two days, enabling comprehensive kinetic screening of a subsequent synthetic step. This rapid adaptability facilitated complete kinetic screening and POC for a complex continuous flow experiment within a single week, a timeframe that is traditionally associated with significantly longer development cycles in conventional process chemistry.
This improved approach to modular continuous flow platform development represents a notable change in the speed and agility of process development and scale-up, particularly within the pharmaceutical industry. The ability to rapidly reconfigure and adapt the platform to different reactor types and reaction conditions significantly reduces development timelines, minimizes material consumption, and accelerates the transition from laboratory-scale experiments to pilot-scale production. This agility is crucial in the fast-paced pharmaceutical industry, where time-to-market is paramount. By streamlining process development and enabling rapid optimization, this modular platform will significantly impact how pharmaceutical companies develop and scale up chemical processes, ultimately leading to faster access to life-saving medications and improved manufacturing efficiency.