2025 AIChE Annual Meeting
(649f) Quantum Corrections to the Kinetic Energy and the Thermodynamic Properties of Cryogenic Fluids
Authors
Richard Sadus - Presenter, Swinburne Univ of Technology
Ulrich K. Deiters, University of Cologne
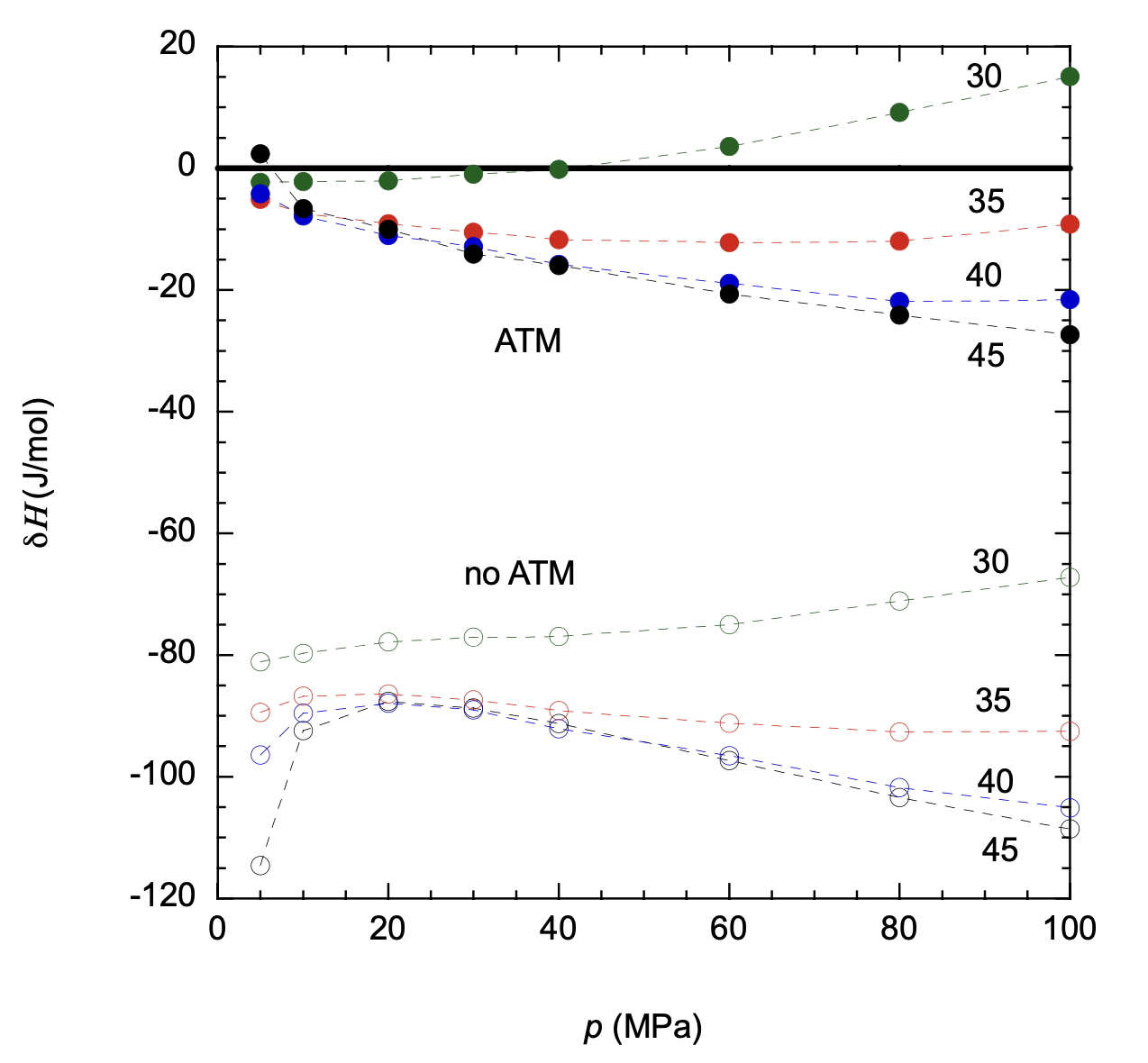
Traditionally the approach to improving the prediction of fluids has focused on obtaining an improved representation of the intermolecular contributions to the potential energy [1]. This has involved developing specialized intermolecular potentials [2] that capture the aspects of interactions arising from such considerations as two-body, three-body forces etc. When used in conjunction with molecular simulations [3], differences in the intermolecular potential are reflected in the calculated thermodynamic properties. This approach is reasonable for normal fluids. However, at cryogenic conditions, some fluids such as hydrogen and neon exhibit a quantum influence on the contribution to the kinetic energy [4], which also affects the caloric thermodynamic properties such as enthalpy, heat capacity, isothermal compressibility, isochoric pressure coefficient and isobaric thermal expansion coefficient. This kinetic energy effect has been largely overlooked in molecular simulations. We have recently observed [5, 6] that accounting for such quantum corrections to the kinetic energy (QCKE) results in predictions that are close in accuracy to reference data. In this talk, we discuss the influence of QCKE on the thermodynamic properties of hydrogen and neon (see example in the figure below) from simulations that use an intermolecular potential that combines ab initio two-body, three-body and quantum terms.
[1] A. J. Stone, The Theory of Intermolecular Forces (Clarendon Press, Oxford, 1996).
[2] U. K. Deiters and R. J. Sadus, J. Chem. Phys. 150, 134504 (2019).
[3] R. J. Sadus, Molecular Simulation of Fluids: Theory, Algorithms, Object-Orientation and Parallel Computing, 2nd Ed. (Elsevier, Amsterdam, 2024).
[4] E. Wigner, Phys. Rev. 40, 749-759 (1932).
[5] U. K. Deiters and R. J. Sadus, J. Chem. Phys. 162, (2025), in press.
[6] U. K. Deiters and R. J. Sadus, (2025), submitted.