2025 AIChE Annual Meeting
(5d) Quantitative Catalyst and Electrolyte Tuning Towards Selective Electrosynthesis Using CO2
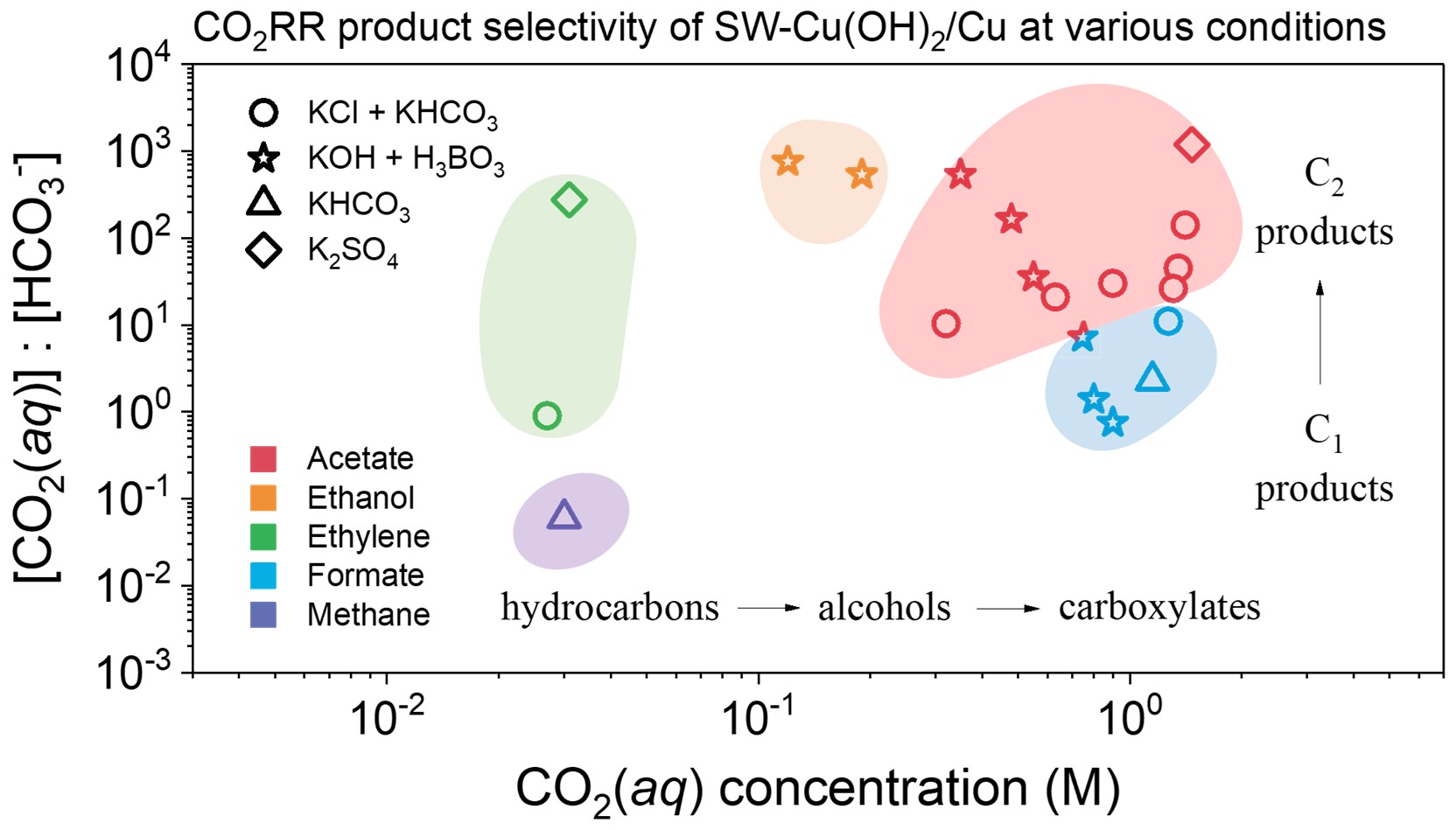
Electrochemical reduction of CO2 (CO2RR) to valuable fuels and chemicals is a promising technique to close the carbon cycle. However, the high complexity in the reaction mechanism leads to a wide product distribution ranging from CO to hydrocarbons, alcohols, carboxylates, and many other oxygenates. Here by experimentally quantifying the availability of electrolytic active species and the evolution of oxide-derived Cu catalysts using various customized electrochemical and spectroscopic characterization techniques, we obtain a statistical trend of CO2RR selectivity applicable to different reaction systems, allowing a quantitative/semi-quantitative tunning of the catalyst and electrolyte parameters that determine CO2RR pathway towards desired kinds of product. Especially, as carboxylates are preferred under CO2 abundant conditions according to the statistics, we achieve record high efficiencies of producing formate/acetate from high-pressure CO2 on oxide-derived Cu catalysts, confirming the reliability of statistical reaction selectivity and indicating a unique bidentate carboxylate pathway. Further efforts in this direction are put into the development of advanced high-throughput/automation electrolysis and characterization methods to expand the database of CO2RR towards more insightful statistics, as well as on the combination of high pressure electrocatalysis with geochemical process in facilitating the mineralization and conversion of stored CO2.