2025 AIChE Annual Meeting
(505d) Purifying mRNA Therapeutics: We Need a Paradigm Shift
Authors
Minimizing the immunogenicity of process- and product-related impurities resulting from in vitro transcription production of ssRNA biologics is crucial. Double stranded RNA (dsRNA), which can vary in length and structure, presents a particular challenge. While the immune system can tolerate low levels of dsRNA in single-dose vaccinations, dsRNA molecules longer than forty base pairs can elicit an innate immune response, necessitating their removal to ensure the safety and efficacy of ssRNA therapeutics. For multiple and larger doses for cancer and other diseases, immunogenic dsRNA cannot be tolerated.
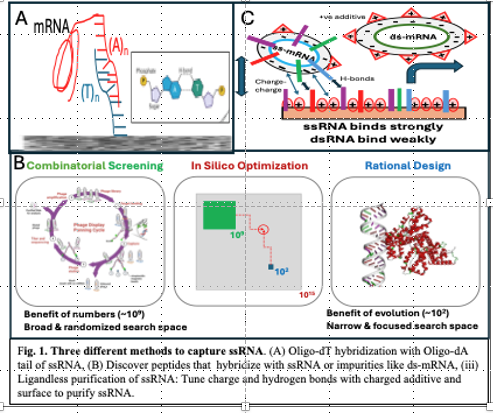
Purification of ssRNA vaccines has saved millions of lives. However, since all purification processes are not 100% efficient, small amounts of immunogenic compounds do leak through with purified ssRNA product causing significant unpleasant immunogenetic responses but not death. Here, we (i) show that, with oligo-dT ligands, convective membrane and monolith processes far outperform chromatographic diffusive processes1, (ii) discover, graft and test our patented selective microporous affinity peptide membranes that selectively bind to ssRNA or dsRNA2, and (iii) present the first details and results from our patented cost-effective ligand-less multimodal surface-modified approach to purify ssRNA from dsRNA3. See Fig. 1.
References
(1) Banik, R.; Neuman, T. G.; Hao, Z.; Al Sharabati M.; Zhao, W.; Anderson., D. G.; Przybycien, T.; Kilduff, J.; Belfort, G. Convection Rather than Diffusion for Fast Efficient mRNA Vaccine Purification. Separation and Purification Tech. 2025, 354.
(2) Hu, M.; Neuman, T. G.; Bai, Y.; Belfort, G.; Karande, P. Discovery and Mechanistic Investigations of RNA-Binding Peptides for Therapeutics Development. submitted 2025.
(3) Neuman, T. G.; Banik, R.; Karla, S.; Hu, M.; Hartin, A.; Karande, P.; Kilduff, J.; Belfort, G. Tuning hydrogen bonds and electrostatics with convection for purifying mRNA: A paradigm shift. Scientific Reports 2025, in press.