2025 AIChE Annual Meeting
(8c) Promotional Effects of CO2 on Pd Catalysts for Direct Synthesis of Hydrogen Peroxide
Authors
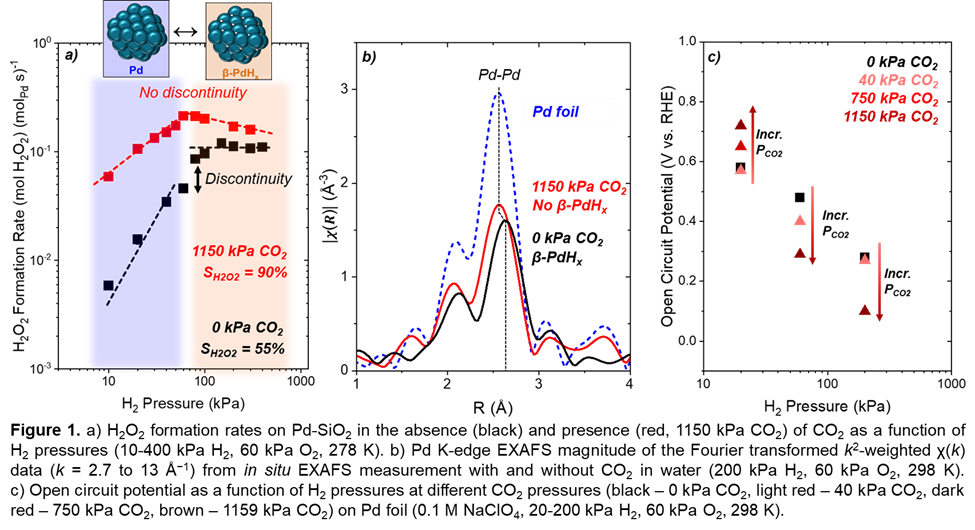
Kinetic measurements reveal CO2 acts as an in situ acid promoter, lowering the reactant solution pH through carbonic acid formation among other potential species and providing additional protons. These changes increase hydrogen coverage on Pd catalysts, doubling H₂O₂ formation rates (Figure 1a). In situ X-ray absorption spectroscopy evidences the suppression of the selective Pd-hydride phase in the presence of CO2 (Figure 1b), yet kinetic measurements reveal an increase in H2O2 selectivity from 55% to 90%. Kinetic isotope effects, combined with in situ infrared spectroscopy and open circuit potentiometry measurements (Figure 1c) confirm that CO2 reduction forms organic residues, which act as surface redox mediators and facilitate proton-electron transfer steps responsible for H2O2 formation. Concurrently, these residues block unselective sites and increase selectivity. Prior studies claim improved H2O2 rates and selectivities with CO2 reflect greater reactant gas solubilities, decreased H2O2 decomposition, and site blocking, however, this work demonstrates CO2 additionally modifies the catalyst structure, surface coverages, and mechanism to collectively result in higher rates and selectivities towards H2O2 on Pd.