2025 AIChE Annual Meeting
(252b) Promoting Alcohol Oxidation over Au Catalysts Via Dilute-Limit Alloying with Rh
Authors
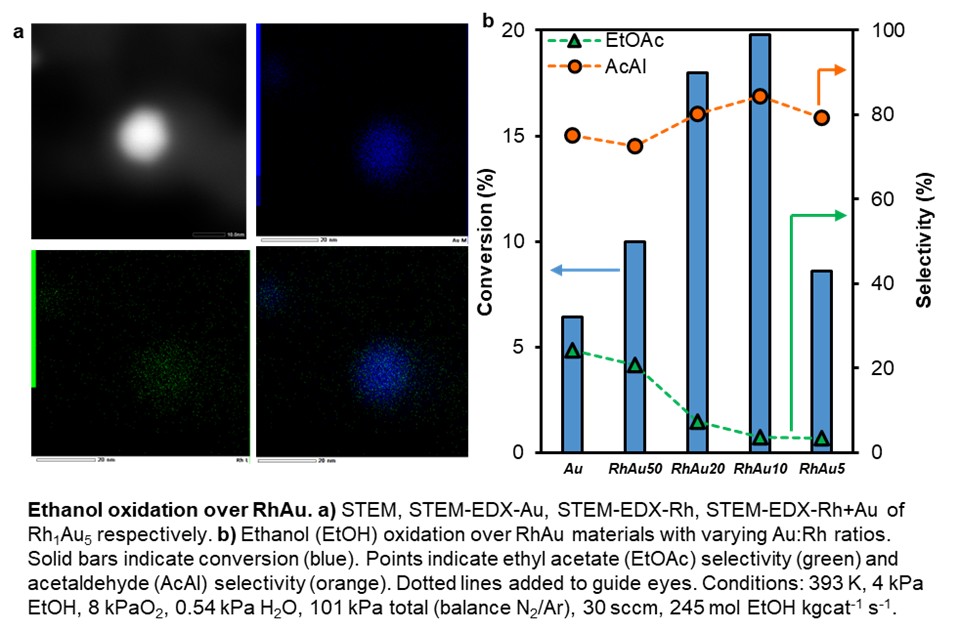
Rh1Aux (x = 5–50) nanoparticles were synthesized via sequential reduction. TEM and EDS confirmed uniform particles (7–9 nm) with Rh enriched locally in Au, and CO-DRIFTS showed Rh remains mostly isolated within Au. Catalytic studies reveal that Rh1Aux promotes ethanol oxidation but with different selectivity than previously observed Pd1Aux systems. While Pd favors oxidative coupling to yield ethyl acetate, increasing Rh content shifts selectivity toward acetaldehyde. This is attributed to differences in ethoxy stabilization versus decomposition: Pd promotes coupling by stabilizing ethoxy species, allowing for facile coupling with acetaldehyde, while Rh stabilizes ethoxy species to a greater extent and triggers comparatively rapid C-H activation, leading to acetaldehyde formation and low ethoxy surface coverages. Acetaldehyde selectivity and oxidation activity increased with Rh content up to a 1:10 Rh:Au ratio, beyond which phase segregation occurred, consistent with Rh–Au immiscibility in bulk. These findings show that Rh doping in SAAs enables tunable control over product distributions, offering design strategies for optimizing activity and selectivity in partial oxidation reactions.