2025 AIChE Annual Meeting
(400au) Process Simulation-Based Evaluation of Internal Stream Recirculation Strategies in Hydrometallurgical Lithium-Ion Battery Recycling Process
Authors
With the accelerating global transition toward renewable energy, there is growing emphasis on sustainability and resource efficiency. As part of this transition, the widespread adoption of lithium-ion batteries (LIBs) in power generation and energy storage systems has significantly increased the demand for critical minerals such as copper, nickel, cobalt, lithium, and rare earth elements—key raw materials for clean energy technologies including batteries, wind turbines, and photovoltaic systems. This increased reliance on LIBs has simultaneously raised concerns regarding the depletion of strategic resources and the management of end-of-life (EoL) battery waste, as the volume of spent LIBs continues to grow at an unprecedented rate [1].
Among various recovery technologies, hydrometallurgical processing has gained particular attention due to its technical maturity, economic feasibility, and high selectivity in recovering target metals [2]. Extensive research has been conducted to advance this hydrometallurgical process for LIB recycling, including recent efforts to estimate associated greenhouse gas emissions. In parallel, numerous studies have compared pyrometallurgical, hydrometallurgical, and direct recycling approaches with respect to technical maturity, process complexity, and energy consumption [3]. However, despite these cross-technology comparisons are well documented, systematic evaluations of alternative process configurations within hydrometallurgical systems have rarely been evaluated from a systematic process design and optimization perspective. In particular, the quantitative impact of internal stream recirculation on chemical consumption, product and byproduct generation, and overall carbon footprint remains largely unexplored, and the associated trade-offs between recovery efficiency, reagent use, and environmental performance have yet to be systematically quantified.
To address these gaps, this study presents a simulation-based comparative evaluation of multiple process configurations incorporating internal stream recirculation. By quantifying material and energy balances and estimating carbon emissions across various scenarios, this work supports the development of environmentally and economically sustainable designs for LIB recycling processes.
Method
In this study, multiple process configurations for hydrometallurgical lithium-ion battery recycling were developed and simulated using Aspen Plus V14. The ELECNRTL property method was employed to represent electrolyte behavior in aqueous-phase reactions and phase equilibria relevant to hydrometallurgical processing. Most unit operations were simulated under ambient conditions, except for a few processes that require specific thermal or pressure settings based on operational data.
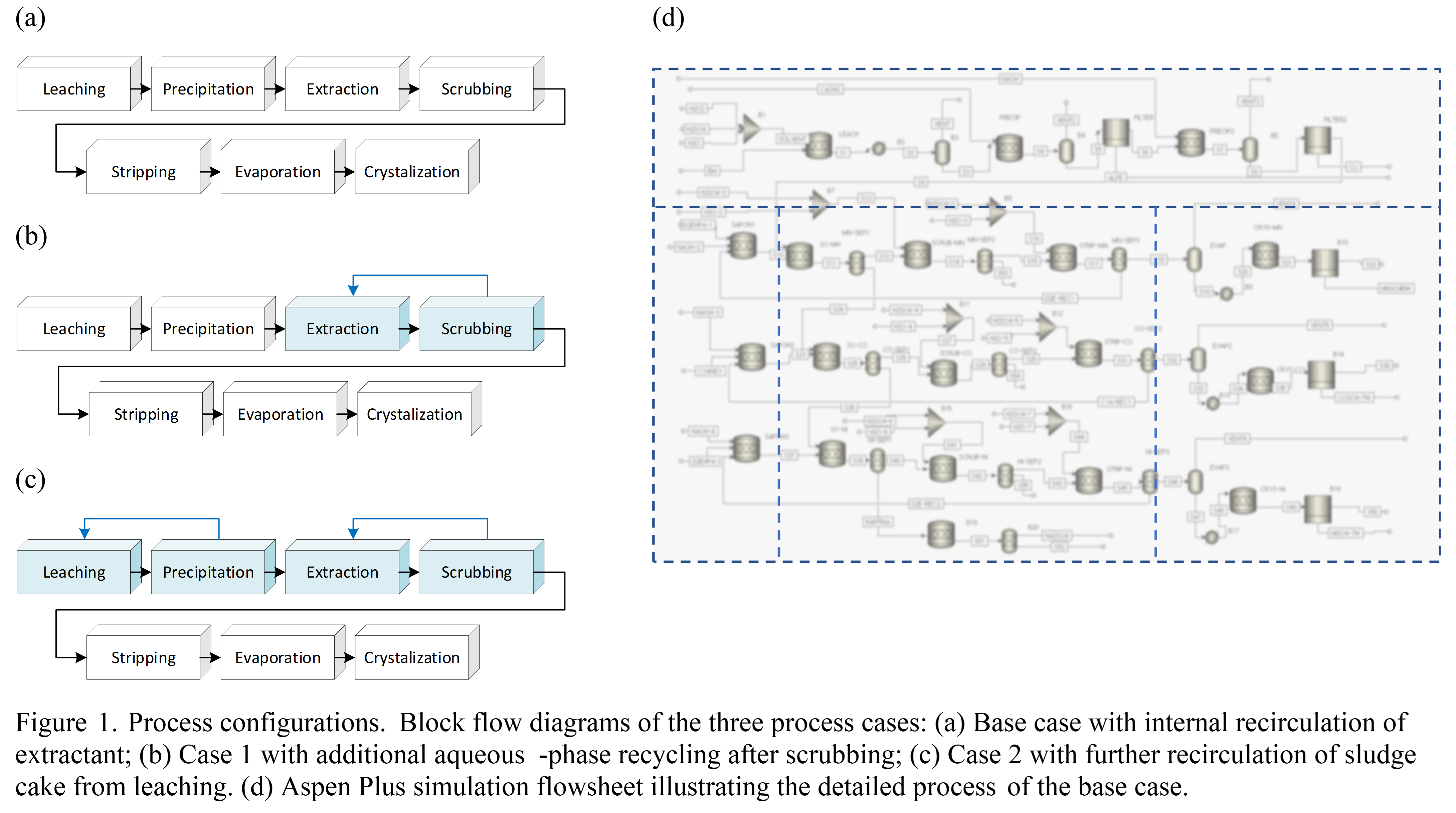
The simulated flowsheet consisted of leaching, solid-liquid separation, solvent extraction (including extraction, scrubbing, and stripping), evaporation, and crystallization units. The feed stream was modeled based on typical black mass composition, containing Co, Ni, Mn, Li, Al, Fe, Cu, and carbon. Stoichiometric reactions were used throughout the process to determine material balances. Material and energy balances were quantified to compare key performance indicators including product yield, chemical consumption, byproduct generation, and CO₂ emissions from electricity use.
Three process scenarios were developed. The base case features internal recirculation of the extractant (organic phase) within the solvent extraction unit. Case 1 introduces additional internal recycling of the main process stream (aqueous phase) following the scrubbing stage. Case 2 incorporates further recirculation of the sludge cake obtained from the solid–liquid separation after leaching. Each configuration was assessed to quantify material and energy balances and to evaluate the effects of internal recirculation strategies on resource consumption and environmental burden within the overall process.
Results
Simulation results indicated that internal stream recirculation has a moderate but notable impact on both process performance and environmental outcomes in hydrometallurgical LIB recycling. In Case 1, the introduction of aqueous-phase recycling after the scrubbing stage led to an improvement in product yield, accompanied by a noticeable increase in the consumption of water, acid, and alkali reagents—particularly within the solvent extraction section. Case 2, which additionally incorporated sludge cake recirculation from the leaching stage, resulted in a slight enhancement in product recovery relative to Case 1, but also intensified reagent usage and operational complexity in the upstream leaching circuit. In both cases, byproduct generation increased appreciably.
One favorable outcome of internal recycling was the modest reduction in extractant (organic phase) consumption, attributed to minimized losses through closed-loop flows in the solvent extraction circuit. On the other hand, increased flow rates due to recycling resulted in greater electricity demands, especially in downstream units such as scrubbing, saponification, and stripping. This, in turn, contributed to elevated indirect CO₂ emissions associated with power consumption.
These findings highlight the trade-offs between enhanced material recovery and increased consumption of chemicals and energy. They underscore the importance of holistically evaluating internal recirculation strategies to inform environmentally and technically balanced process design.
Conclusion
This study provides a simulation-based quantitative evaluation of internal recirculation strategies in hydrometallurgical recycling of spent lithium-ion batteries. By analyzing multiple process configurations using Aspen Plus, the work highlights key trade-offs between product recovery, chemical consumption, and environmental emissions, offering valuable insights for early-stage process design. The integrated approach enabled the assessment of material and energy balances, comparison of internal stream recycling strategies, and estimation of electricity-derived carbon emissions across scenarios. These outcomes contribute to the development of more environmentally and economically sustainable LIB recycling systems and underscore the importance of system-level design optimization.
Future work will include sensitivity studies on key process variables to identify critical trade-off conditions in process design. In addition, the simulation model will be refined by incorporating reactor types that reflect chemical equilibrium behavior, rather than relying on fixed conversion rates, to enhance model accuracy and reliability.
Acknowledgement
This research was supported by the Basic Research Project (25-3223) of the Korea Institute of Geoscience and Mineral Resources(KIGAM) funded by the Ministry of Science and ICT of Korea. It is also supported by the Technology development project to improve secondary battery circulation suability (Development of pollutants reduction technology generated in the lithium ion batteries recycling process) through the Korea Environmental Industry & Technology Institute funded by the Ministry of Environment(RS-2024-00345911).
References
[1] Agency, I. E. Recycling of Critical Minerals: Strategies to Scale Up Recycling and Urban Mining, 2024.
[2] Jia, L.-p.; Huang, J.-j.; LIU, X.-h.; CHEN, X.-y.; LI, J.-t.; HE, L.-h.; ZHAO, Z.-w. Research and development trends of hydrometallurgy: An overview based on Hydrometallurgy literature from 1975 to 2019. Transactions of Nonferrous Metals Society of China, 2020, 30(11), 3147-3160.
[3] Jung, J. C.-Y.; Sui, P.-C.; Zhang, J. A review of recycling spent lithium-ion battery cathode materials using hydrometallurgical treatments. Journal of Energy Storage, 2021, 35, 102217.