2025 AIChE Annual Meeting
(503c) Process Simulation and Multi-Aspect Analysis of Methanol and Olefin Production from Steel Mill Gases
Authors
Ki Woong Kim - Presenter, KRICT
Ji Woong Yoon, Korea Research Institute of Chemical Technology
Ji Sub Yun, University of Ottawa
Jaedeuk Park, Korea Research Institute of Chemical Technology
Kosan Roh, Korea Advanced Institute of Science and Technology (KAIST)
The steel-making industry primarily depends on the blast furnace process, where carbon removes oxygen from iron ore. Currently, about 70% of global steel production follows the Blast Furnace–Basic Oxygen Furnace (BF-BOF) route, with over 90% of CO₂ emissions originating from three key steel mill gases: Blast Furnace Gas (BFG), Coke Oven Gas (COG), and Basic Oxygen Furnace Gas (BOFG) [1]. However, electricity generation from these gases is notably carbon-intensive—up to three times higher than that of pulverized coal power plants [2]. This inefficiency stems mainly from the heavy reliance on BFG, which has the lowest calorific value among the three gases, resulting in poor energy conversion performance.
To address this, transitioning electricity generation to cleaner energy sources while redirecting BFG for alternative process uses could improve both energy efficiency and sustainability. Moreover, CO₂ emissions could be reduced by approximately 7% if natural gas were used for heat generation within the plant instead of steel mill gases [3]. Additional reductions may be achievable through the adoption of electric heating technologies, though these have not yet been scaled for industrial deployment. Achieving these improvements will require the development of alternative strategies to manage steel mill exhaust gases and to effectively utilize the carbon they contain.
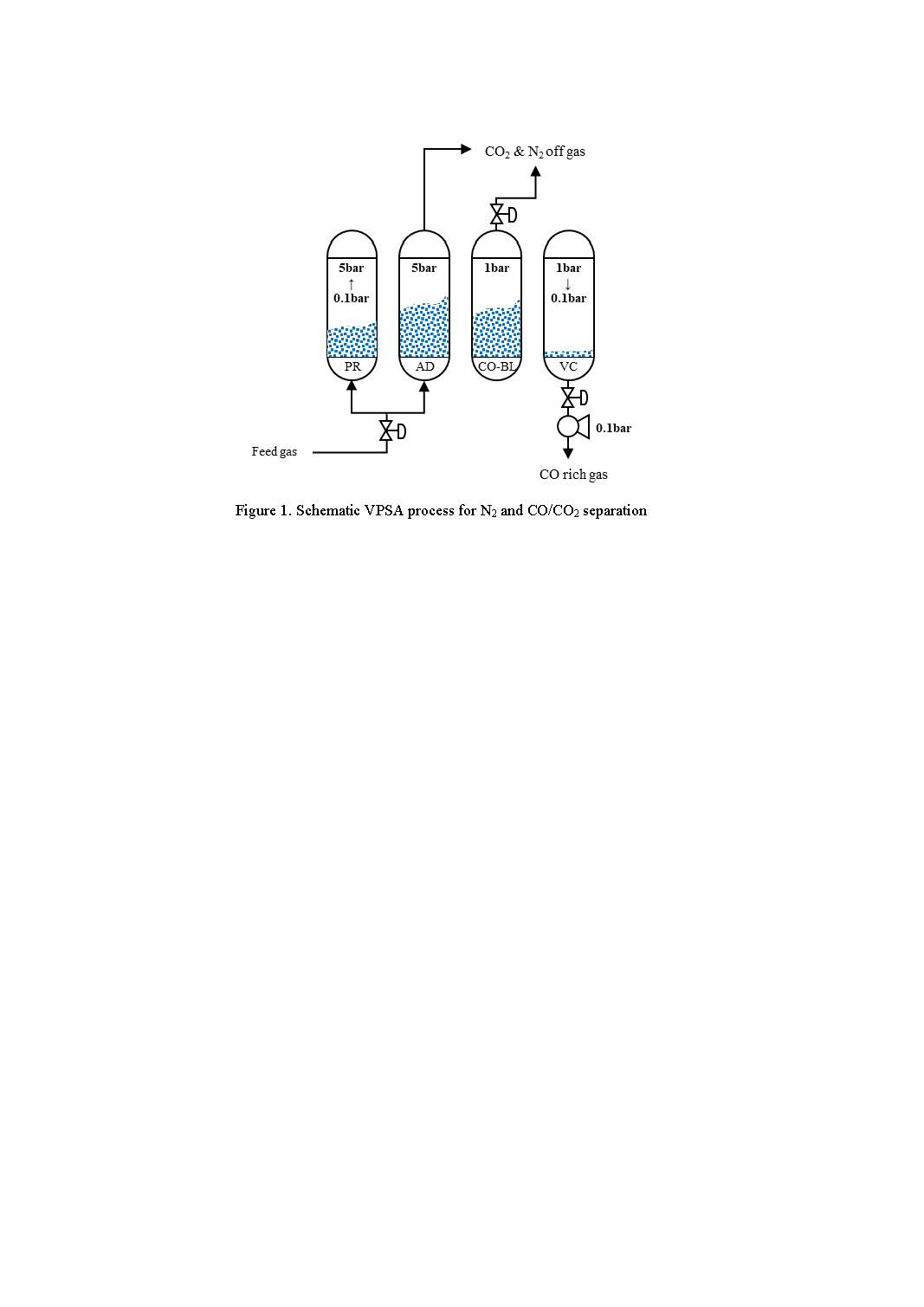
We introduce a proposed process that produce methanol and olefins from the feed of BFG and LNG. To synthesize methanol, carbon rich gas which is primarily CO recovered from BFG is processed through a series of integrated separation processes, including membrane contactors, gas separation membranes, and vacuum pressure swing adsorption (VPSA). The recovered CO is subsequently blended with syngas produced via steam methane reforming (SMR) from LNG feedstock. This syngas is then converted into methanol, which serves as a feedstock for light olefin production via the naphtha-methanol-to-olefin (NMTO) process. Compared to conventional steam naphtha cracking (SNC), the NMTO route offers enhanced energy efficiency, requiring approximately 30% less thermal input for equivalent olefin yields, thereby contributing to both process intensification and reduced carbon footprint.
Based on the sensitivity analysis, the VPSA unit for CO separation was identified as one of the primary contributors to overall energy consumption and CO₂ emissions. An additional comparative analysis was conducted using two different adsorbents. These adsorbents impregnated copper metal onto different supporters. The adsorption equilibrium and mass transfer coefficient were characterized by experimental data such as adsorption isotherm and breakthrough. One is zeolite and the other one is boehmite. The sensitivity analysis was conducted to find out the optimum operating parameters of VPSA process and the overall process performance will be analyzed.
To address this, transitioning electricity generation to cleaner energy sources while redirecting BFG for alternative process uses could improve both energy efficiency and sustainability. Moreover, CO₂ emissions could be reduced by approximately 7% if natural gas were used for heat generation within the plant instead of steel mill gases [3]. Additional reductions may be achievable through the adoption of electric heating technologies, though these have not yet been scaled for industrial deployment. Achieving these improvements will require the development of alternative strategies to manage steel mill exhaust gases and to effectively utilize the carbon they contain.
We introduce a proposed process that produce methanol and olefins from the feed of BFG and LNG. To synthesize methanol, carbon rich gas which is primarily CO recovered from BFG is processed through a series of integrated separation processes, including membrane contactors, gas separation membranes, and vacuum pressure swing adsorption (VPSA). The recovered CO is subsequently blended with syngas produced via steam methane reforming (SMR) from LNG feedstock. This syngas is then converted into methanol, which serves as a feedstock for light olefin production via the naphtha-methanol-to-olefin (NMTO) process. Compared to conventional steam naphtha cracking (SNC), the NMTO route offers enhanced energy efficiency, requiring approximately 30% less thermal input for equivalent olefin yields, thereby contributing to both process intensification and reduced carbon footprint.
Based on the sensitivity analysis, the VPSA unit for CO separation was identified as one of the primary contributors to overall energy consumption and CO₂ emissions. An additional comparative analysis was conducted using two different adsorbents. These adsorbents impregnated copper metal onto different supporters. The adsorption equilibrium and mass transfer coefficient were characterized by experimental data such as adsorption isotherm and breakthrough. One is zeolite and the other one is boehmite. The sensitivity analysis was conducted to find out the optimum operating parameters of VPSA process and the overall process performance will be analyzed.
1. Iron and Steel Technology Roadmap. 2020, IEA: Paris.
2. Grigsby-Schulte, C.S.a.A., Pedal to the Metal 2023: Time to Shift Steel Decarbonization into High Gear. 2023, Global Energy Monitor.
3. Mission Possible: Reaching net-zero carbon emissions from harder-to-abate sectors by mid-century. 2018, Energy Transitions Commission.