2025 AIChE Annual Meeting
(501g) Process Design and Optimization for Green Ammonia Production
- Modelling of the green ammonia production process in a steady-state process simulator;
- Quantification of mass and energy balances and assessment of Key Performance Indicators (KPIs);
- Sensitivity analysis to identify optimal process configuration (electrolyser type, operating pressure);
- LCOA for the optimized process.
An industrial-relevant electrolyser scale of 500 MW is considered as the process size.
Model structure
Submodels have been developed to characterize the building blocks involved in green ammonia synthesis, namely Air Separation Unit (ASU), Proton Exchange Membrane (PEM) and Solid Oxide Electrolyser Cell (SOEC) electrolysers, and the ammonia synthesis reactor. A rigorous model approach is followed for both the ammonia synthesis reactor and ASU. The conventional Haber-Bosch model is largely based on the process description reported in Ullmann’s encyclopaedia [4]. The main assumptions are:
- operating pressure is 210 bar;
- three packed beds in series with intermediate cooling. Inlet temperature to each bed is 380°C;
- kinetics of forward and reverse reactions retrieved from Burrows and Bollas [5];
- ammonia recovery by liquefaction in two steps operating at 20°C and 0°C, respectively.
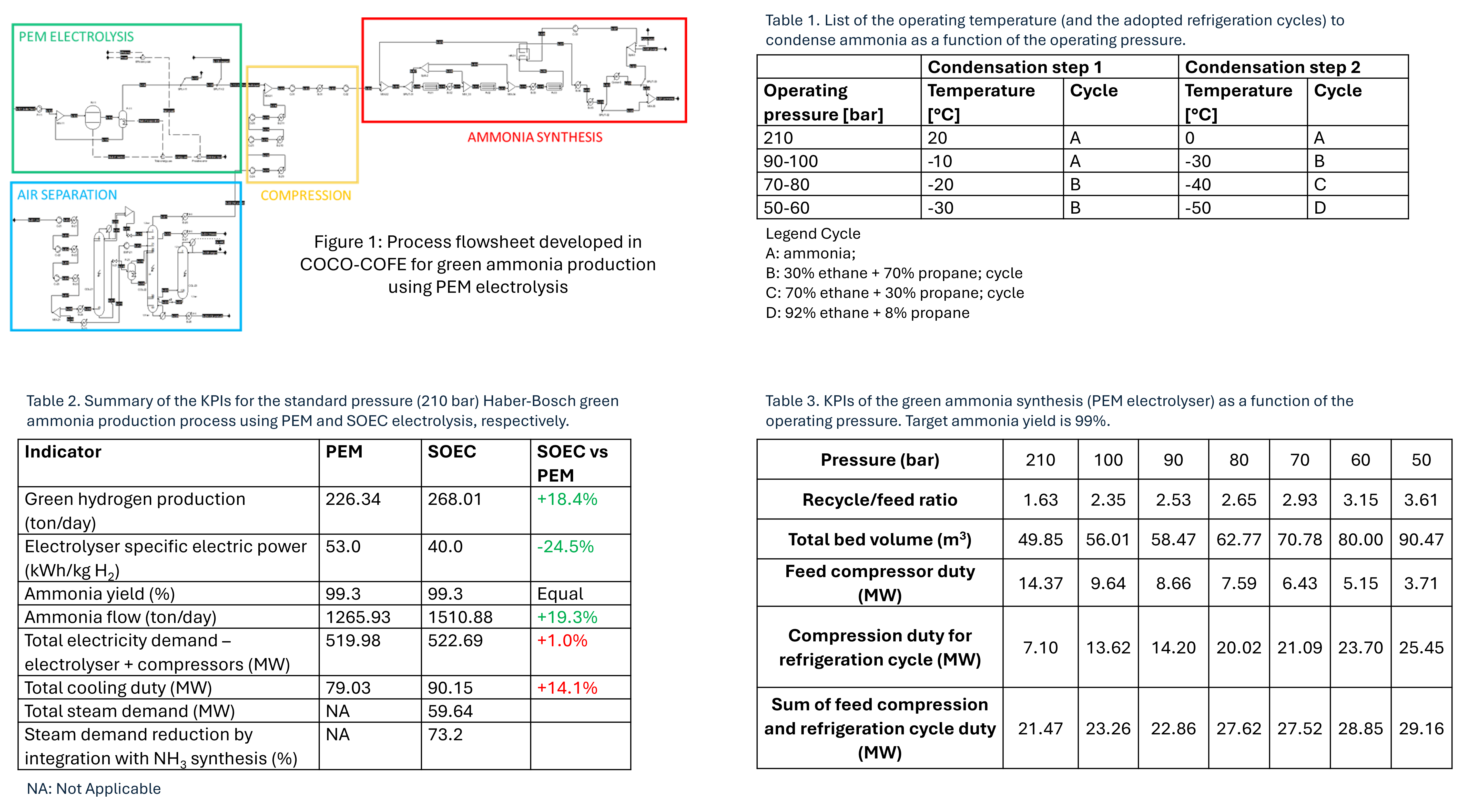
For the low-pressure process, a pressure range between 50 and 100 bar is investigated. The kinetics for the ruthenium catalyst is retrieved from Yoshida et al. [6]. At reduced pressure, lower temperatures are needed to condense ammonia. The major implication is the need to replace the ammonia refrigeration cycle considered for Haber-Bosch with alternative refrigerants. A list of the adopted cycles for different operating pressures is available in Table 1.The electrolyser is represented with a simplified soft model accounting only for the global water splitting reaction, and the corresponding efficiencies and performance indicators are directly retrieved from a previously published work [7].Flowsheet is developed in COCO-COFE (CAPE-OPEN to CAPE-OPEN – CAPE-OPEN Simulation Environment), a simulation package available free of charge. The Peng-Robinson Equation of State is used as the thermodynamic model. The flowsheet of the integrated process is drawn in Figure 1 (for the PEM case-study).
Costs assessment
Costs will be calculated through a class III estimate. Mass and energy balances from the COFE model are used for unit sizing and to quantify raw materials and utilities consumption. CAPEX and OPEX will be determined using published correlations [8,9].
Sensitivity analysis
- Performances of green ammonia production at conventional pressure using PEM and SOEC are compared in terms of KPIs and LCOA.
- A sensitivity analysis is carried out to investigate how energy requirements and reactor size change when operating with Ru-catalyst at different pressures between 50 and 100 bar. The target is to find the optimal pressure and compare low-pressure process with conventional Haber-Bosch.
Results
Table 2 compares the KPIs for the conventional Haber-Bosch process integrated with PEM and SOEC electrolysers, respectively. Results show that SOEC and PEM provide the same ammonia yield. However, SOEC allows generating a higher ammonia flow (+19%) thanks to the higher hydrogen production at given electrolyser power (500 MW). On the other hand, the increase in the total electricity demand observed for SOEC is limited to 1%. Noteworthily, more than 70% of the steam needed for SOEC can be produced through heat integration using the residual heat available from the hot streams leaving each packed bed in ammonia synthesis (T > 450°C). Table 3 reports a summary of the main KPIs for the low-pressure process using PEM electrolysers.
The following considerations can be drawn:
- At lower pressure, lower temperatures are required to condense ammonia. This results in a considerable increase in the electricity requirement of the refrigeration cycle.
- The lower the pressure, the higher the flow of unconverted reactants to be recycled. Indeed, the thermodynamic equilibrium of NH3 production becomes less favorable, so the conversion per pass decreases.
- The reaction kinetics is slower at lower pressure.
- Circulating flows inside the reactor are higher, thus higher packed bed heights are needed to achieve the same yield.
- Working at lower pressures considerably drops the cost for make-up gas compression.
- The optimal pressure range for the low-pressure ammonia synthesis is 90 bar. The total electricity demand is only +6.5% compared to conventional Haber-Bosch.
Similar considerations hold for the SOEC-based low-temperature process. Calculations of the LCOA for PEM and SOEC-based processes at both 210 bar (conventional) and 90 bar (optimized low-pressure) are in progress and will be presented at the meeting. This will be crucial to assess the economic feasibility of the proposed integrated ammonia production process.
Acknowledgement
The present work received financial support from the Norwegian Research Council’s Centres for Environment-friendly Energy Research program.
References
[1] B. Lee, L.R. Winter, H. Lee, D. Lim, H. Lim, M. Elimelech, Pathways to a Green Ammonia Future, ACS Energy Lett (2022) 3032–3038. https://doi.org/10.1021/ACSENERGYLETT.2C01615/ASSET/IMAGES/MEDIUM/NZ2C0….
[2] A.G. Olabi, M.A. Abdelkareem, M. Al-Murisi, N. Shehata, A.H. Alami, A. Radwan, T. Wilberforce, K.J. Chae, E.T. Sayed, Recent progress in Green Ammonia: Production, applications, assessment; barriers, and its role in achieving the sustainable development goals, Energy Convers Manag 277 (2023) 116594. https://doi.org/10.1016/J.ENCONMAN.2022.116594.
[3] C. Arnaiz del Pozo, S. Cloete, Techno-economic assessment of blue and green ammonia as energy carriers in a low-carbon future, Energy Convers Manag 255 (2022) 115312. https://doi.org/10.1016/J.ENCONMAN.2022.115312.
[4] K.H.R. Rouwenhorst, P.M. Krzywda, N.E. Benes, G. Mul, L. Lefferts, Ammonia, 4. Green Ammonia Production, in: Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, 2020.
[5] L. Burrows, G.M. Bollas, Stability Assessment of Small-Scale Distributed Ammonia Production Systems, Ind Eng Chem Res 61 (2022) 16081–16092. https://doi.org/10.1021/ACS.IECR.2C00631/SUPPL_FILE/IE2C00631_SI_001.PDF.
[6] M. Yoshida, T. Ogawa, Y. Imamura, K.N. Ishihara, Economies of scale in ammonia synthesis loops embedded with iron- and ruthenium-based catalysts, Int J Hydrogen Energy 46 (2021) 28840–28854. https://doi.org/10.1016/J.IJHYDENE.2020.12.081.
[7] A. Zaccara, A. Petrucciani, I. Matino, T.A. Branca, S. Dettori, V. Iannino, V. Colla, M. Bampaou, K. Panopoulos, Renewable Hydrogen Production Processes for the Off-Gas Valorization in Integrated Steelworks through Hydrogen Intensified Methane and Methanol Syntheses, Metals 2020, Vol. 10, Page 1535 10 (2020) 1535. https://doi.org/10.3390/MET10111535.
[8] K.M. Guthrie, Process Plant Estimating, Evaluation and Control, Solana Ed., 1974.
[9] R. Turton, J.A. Shaewitz, D. Bhattacharya, W.B. Whiting, Analysis, Synthesis, and Design of Chemical Processes, Fifth Edition, Pearson, 2018.