2025 AIChE Annual Meeting
(332c) Probing Solid-Fluid Interactions in Polymer Particle Suspensions Using Time-Domain NMR
Authors
Alan Allgeier - Presenter, University of Kansas
Murilo Toledo Suekuni, The University of Kansas
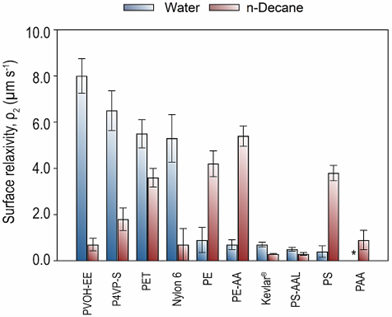
Low-field time-domain nuclear magnetic resonance (NMR) data characterize wetted surface area in particle suspensions but there is a dearth of insight in the literature on quantitative correlations between surface chemistry and perturbations in solvent nuclear magnetic spin relaxation. Polymer particle suspensions were prepared with ground copolymers of known surface chemistry in water and n-decane. Trends in solvent transverse relaxation rates demonstrated that surface polar functional groups induce stronger interactions with water, with the opposite effect for n-decane. NMR surface relaxivities (ρ2) calculated for the solid-fluid pairs ranged from 0.4 to 8.0 μm s-1 and 0.3 to 5.4 μm s-1 for water and n-decane, respectively. The values of ρ2 for water displayed an inverse relationship to contact angle measurements on surfaces of similar composition from the literature, supporting the correlation of the TD-NMR output to polymer wettability. Surface composition, i.e., H/C ratios and heteroatom content, mainly contributed to the observed surface relaxivities, compared to polymer percent crystallinity and mean particle sizes via multiple linear regression. Ultimately, these findings emphasize the significance of surface chemistry in TD-NMR measurements and serve as a reference for future investigations involving complex systems with similar chemical properties. A comprehensive understanding of the factors influencing solvent relaxation in porous media can aid the optimization of industrial processes and the design of materials with enhanced performance.