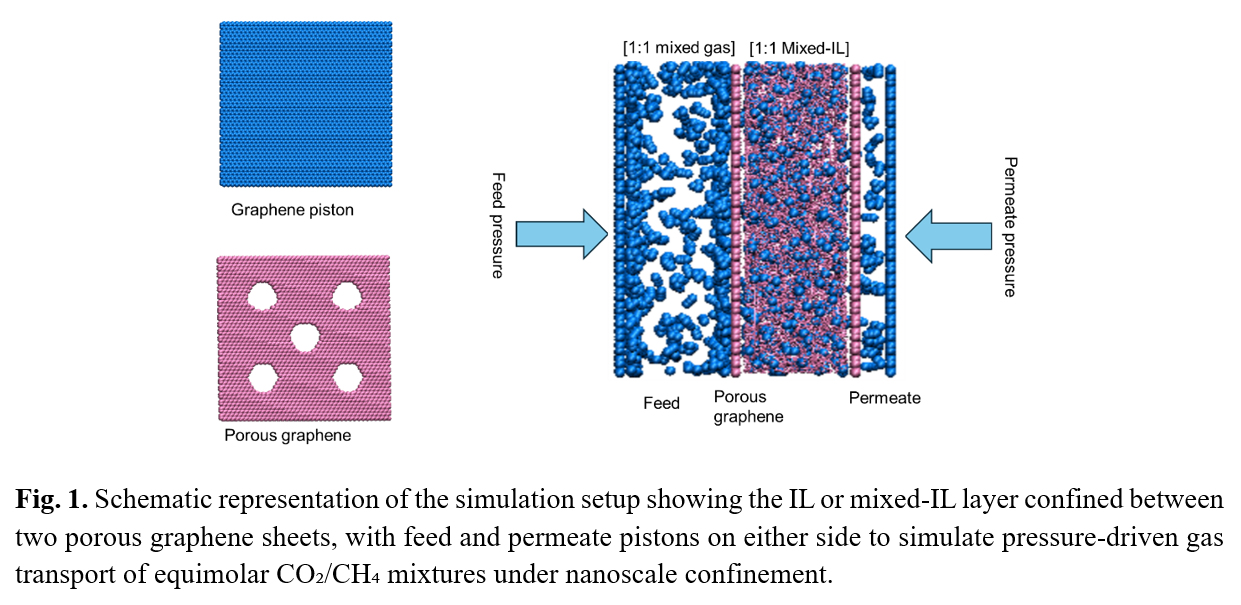
The strategic confinement of ionic liquids (ILs) in advanced membrane systems, such as copolymer matrices and porous graphene, has reportedly enhanced gas separation performance by modifying diffusivity, solubility, and selectivity. Recent studies highlight two key advancements in this area. Firstly, microphase-separated ILs in block copolymer matrices (e.g., segregating between two polymer phases) improves gas transport more effectively than distributing it in a distinct polymer phase. This is attributed to optimized interfacial interactions and tailored diffusion pathways [1]. Secondly, binary mixtures of ILs, particularly those sharing a common cation but differing in anion structure, enable tunable control over key properties such as viscosity, molar volume, and free volume, which directly influence CO
2 solubility and selectivity [2]. The synergy between different anionic characters in the mixtures tailors the physical and chemical interactions with specific gas molecules, such as CO
2 and CH
4 [3]. While prior studies have independently explored either mixed IL systems or nanoconfined ILs, their intersection remains largely unexamined. In particular, the gas separation behavior of binary IL mixtures under nanoscale confinement, where both molecular structure and spatial restrictions critically influence transport, has not yet been systematically investigated. For instance, computational models of confined ILs focus on single IL systems, neglecting how mixed-IL chemistry could amplify CO
2 selectivity under confinement [1]. Conversely, experimental investigations of IL mixtures prioritize bulk properties, overlooking confinement-driven phenomena such as capillary freezing or layered nanostructures that could dynamically modulate gas transport [4]. This disconnect hinders the design of membranes that synergistically combine mixed-IL tunability with nanoconfinement advantages. Addressing this gap could unlock new design principles for tailoring high-performance membranes, especially in supported configurations like porous graphene that stabilize the IL phase, while maintaining accessible pathways for selective gas diffusion. In this work we utilize porous graphene, which offers superior mechanical strength and chemical stability, as a structural support to study gas transport and separation properties in supported mixed ionic liquids (mixed-IL) membranes, aiming to enhance the unique interactions at the interface of graphene and IL mixture for improved gas separation performance. Three systems were simulated, including [EMIM][TF2N], [EMIM][Cl] and a mixture of [EMIM][TF2N] and [EMIM][Cl] at an equimolar ratio, confined by porous graphene. The simulation setup involved an IL mixture sandwiched between two porous graphene sheets, with equimolar CO
2/CH
4 gas feeding implemented through pressurized piston-driven assembly, as shown in Fig. 1. Molecular dynamics (MD) simulations were performed on the system elements built in the BIOVIA Materials Studio software, using the open-source large-scale atomic/molecular massively parallel simulator (LAMMPS). The OPLS-AA forcefield was used to describe the molecular interactions in the ILs and CH
4, while the EPM2 model was used for CO
2. The simulation protocol involved a 5-ns NPT equilibration run, followed by another 5-ns NVT production run at 298 k to calculate gas flux, permeance, diffusivity, solubility, and selectivity of CO
2 over CH
4. Our objective was to investigate how the IL composition and nanoconfinement geometry influences gas transport and separation performance. We hypothesized that confining a binary IL mixture, composed of [EMIM][TF₂N] and [EMIM][Cl], between porous graphene sheets would synergistically combine the high CO
2 affinity of [TF₂N]⁻ with the distinctive physicochemical traits of [Cl]⁻, resulting in enhanced transport and separation performance beyond that of either pure IL. We are currently analyzing and verifying our results on fully equilibrated pure and mixed-IL systems with experimental observations. We will present our results in terms of gas flux, permeance, diffusivity, selectivity, density profiles, and mean square displacement (MSD) plots. This research highlights the promise of graphene-supported IL membranes for high-performance gas separation, with a particular focus on CO
2 capture. The findings from this study provide a strong foundation for advancing the scalable implementation of these membranes in industrial applications.
References:
[1] A. Salmankhani, A. M. Lopez, P. Scovazzo, A. E. Smith, and S. Nouranian, “Molecular Simulation of CO2/CH4 Transport and Separation in Polystyrene-block-poly(ethylene oxide)/Ionic Liquid (IL) Membranes: Insights into Nanoconfined IL Effects.,” ACS Appl Mater Interfaces, vol. 17, no. 7, pp. 11348–11361, doi: 10.1021/acsami.4c21064.
[2] L. C. Tomé, D. Patinha, C. Freire, L. P. N. Rebelo, and I. Marrucho, “CO2 separation applying ionic liquid mixtures: The effect of mixing different anions on gas permeation through supported ionic liquid membranes,” RSC Adv., vol. 3, Jun. 2013, doi: 10.1039/C3RA41269E.
[3] A. M. Pinto, H. Rodríguez, Y. J. Colón, A. Jr. Arce, A. Arce, and A. Soto, “Absorption of Carbon Dioxide in Two Binary Mixtures of Ionic Liquids,” Ind. Eng. Chem. Res., vol. 52, no. 17, pp. 5975–5984, doi: 10.1021/ie303238h.
[4] F. D’Anna, S. Marullo, P. Vitale, and R. Noto, “Binary Mixtures of Ionic Liquids: A Joint Approach to Investigate their Properties and Catalytic Ability,” ChemPhysChem, vol. 13, no. 7, pp. 1877–1884, 2012, doi: https://doi.org/10.1002/cphc.201100878.