2025 AIChE Annual Meeting
(526f) Predicting Branched Hydrogenolysis Mechanisms on Ru and Ir Surfaces from Model Compound Activations on Crowded H-Covered Metals
Authors
Andy Simonson - Presenter, University of Florida
Samantha Carozzi, Georgia Institute of Technology
David Hibbitts, University of Florida
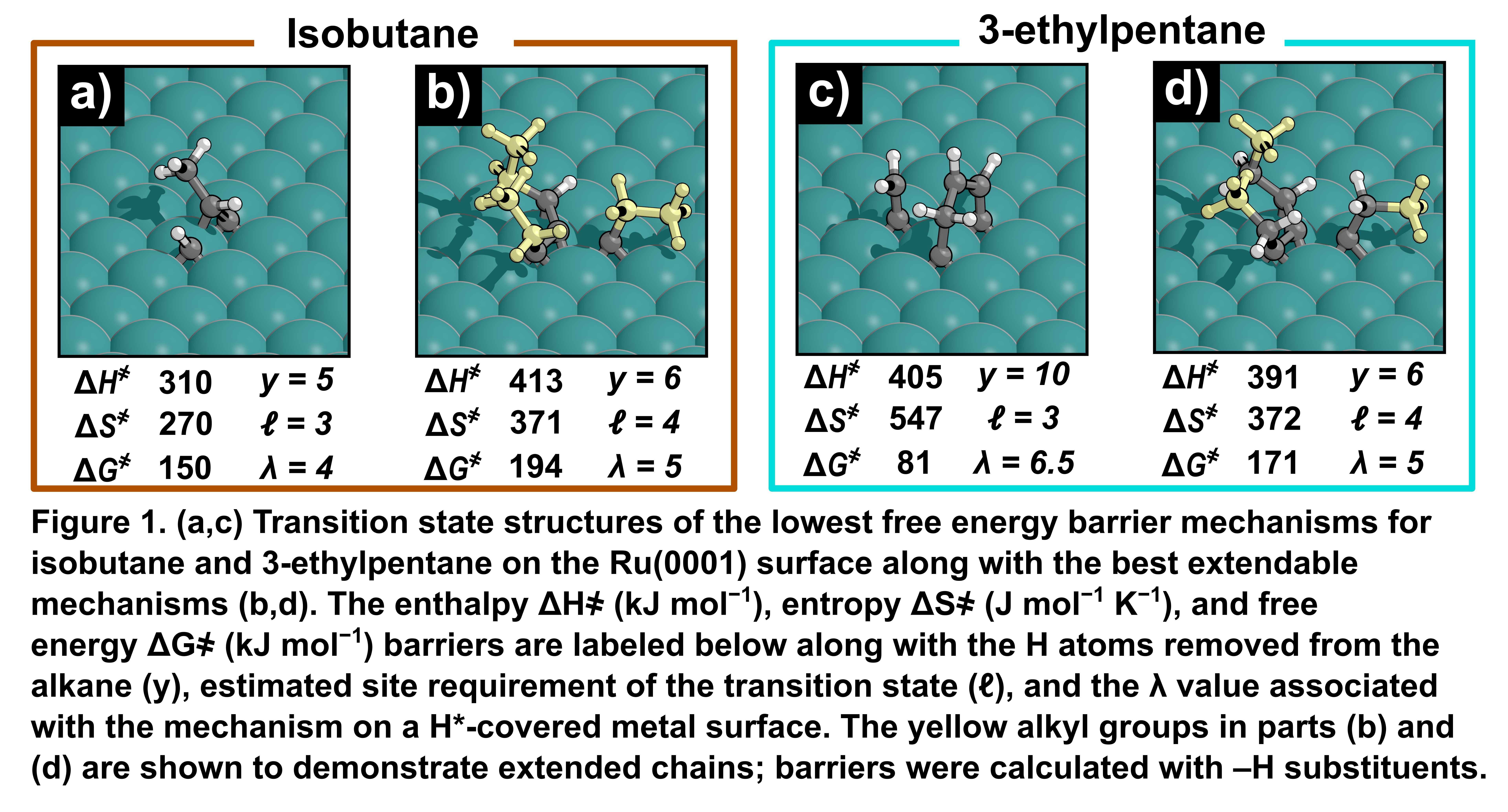
C–C hydrogenolysis chemistry has gained attention as a method to chemically upcycle polyolefins resistant to traditional polymer recycling methods. Gas-phase C–C hydrogenolysis of alkanes on H*-covered Ir surfaces has been extensively studied, resulting in an understanding of the activation mechanisms and hydrogen pressure dependencies of unsubstituted and substituted activations in alkanes. Hydrogenolysis on H*-covered metals requires the removal of H from both the surface, to fit the alkane-derived transition state within the crowded adlayer (ℓ), and from the alkane, prior to the rate-determining C–C scission elementary step (y), evolving λ molecules of H2 gas. Experimental results from substituted alkanes indicate that methyl-substituted alkane activations involve transition states that evolve more H2 compared to unsubstituted activations while supplementary computational results indicate that adjacent methyl groups play a critical role in activations at branch points, being extensively dehydrogenated such that analogous activations of larger branches, present in 3-ethylpentane, would be impossible. These results are in direct contrast to unsubstituted activations where C2 to C10 linear alkanes activate through analogous transition states. Preliminary results of isobutane hydrogenolysis on bare Ir(111) and Ru(0001) surfaces indicate that activations primarily occur through isobutane-derived intermediates requiring methyl substituents adjacent to the cleaved bond; however, “extendable” isobutane activations, where every terminal C atom retains at least one H (e.g. Fig. 1b), can analogously occur in branches with substituents larger than a methyl group. While preliminary data shows extendable activations of isobutane and 3-ethylpentane are generally less favorable than non-extendable activations on bare surfaces, crowded surfaces penalize transition states covering large areas of the metal surface. Preliminary results on H*-covered surface models indicate that isobutane primarily activates through extendable mechanisms transferable to larger branched compounds. Contrasting isobutane and 3-ethylpentane activations in crowded adlayers may give insight into a generalized mechanism of branched hydrogenolysis transferable to polyolefins.