2025 AIChE Annual Meeting
(184i) Porous Liquid Crystalline Polymer Scaffolds for 3D Cell Culture
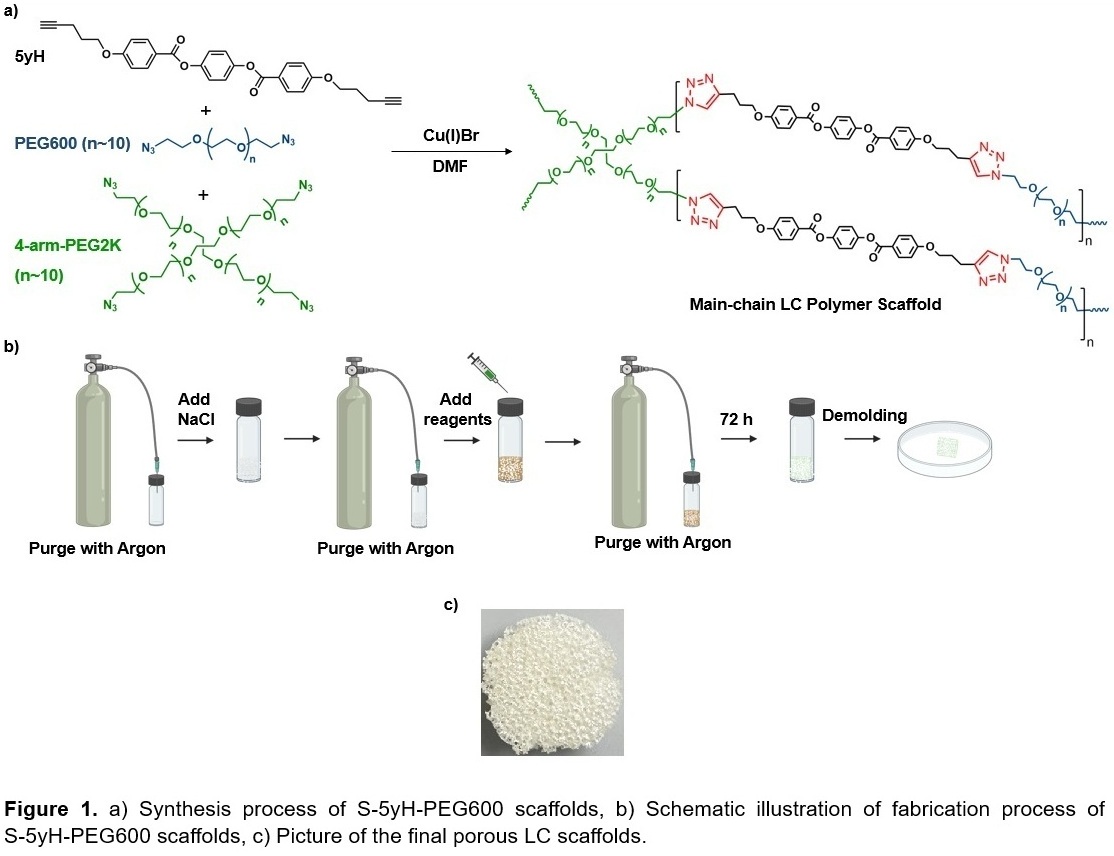
The scaffolds (S-5yH-PEG600) were synthesized using a one-step copper-catalyzed alkyne-azide cycloaddition (CuAAC) reaction between dialkyne-functionalized LC mesogens (5yH), diazide-terminated polyethylene glycol (PEG600) oligomers, and tetrafunctional azide polyethylene glycol (4arm-PEG2k) crosslinkers. To generate porosity within the networks, a salt-leaching technique was used. This method enables control over the pore size and structure of polymeric scaffolds to ensure sufficient space for cells to attach, migrate, and receive nutrients. Unlike other LC scaffolds that are primarily side-chain LC materials, this approach generated main-chain LC networks, which have the potential for stronger coupling of LC order to polymer chain confirmation compared to side-chain LC polymers.
The resulting networks showed high gel fractions, indicating good reaction efficiency. Differential Scanning Calorimetry (DSC) verified a sub-ambient glass transition and an LC phase transition. Polarized Optical Microscopy (POM) showed birefringence due to the presence of a liquid crystalline phase. Scanning Electron Microscopy (SEM) confirmed uniformity, connectivity, and distribution of pores within the structure. Mechanical testing demonstrated that these materials have adjustable stiffness, and shape-morphing properties, including actuation, which is a reversible extension and contraction of the material in response to stimuli. The networks also supported the attachment and growth of human mesenchymal stem cells.
Overall, this work demonstrates a new strategy for fabricating porous and biodegradable LC polymer scaffolds. These materials have both the benefits of LC anisotropy and tunable porosity, making them excellent materials for advanced 3D cell culture and tissue engineering applications.
Reference:
[1] Wang, Y.; Burke, K. A. Soft Matter Phase Behavior of Main-Chain Liquid Crystalline Polymer Networks Synthesized by Alkyne – Azide Cycloaddition Chemistry. Soft Matter 2018, 14 (48), 9885–9900.