2025 AIChE Annual Meeting
(536d) Porous Electrode Theory Using Phase Field Modeling & Reaction Engineering
Authors
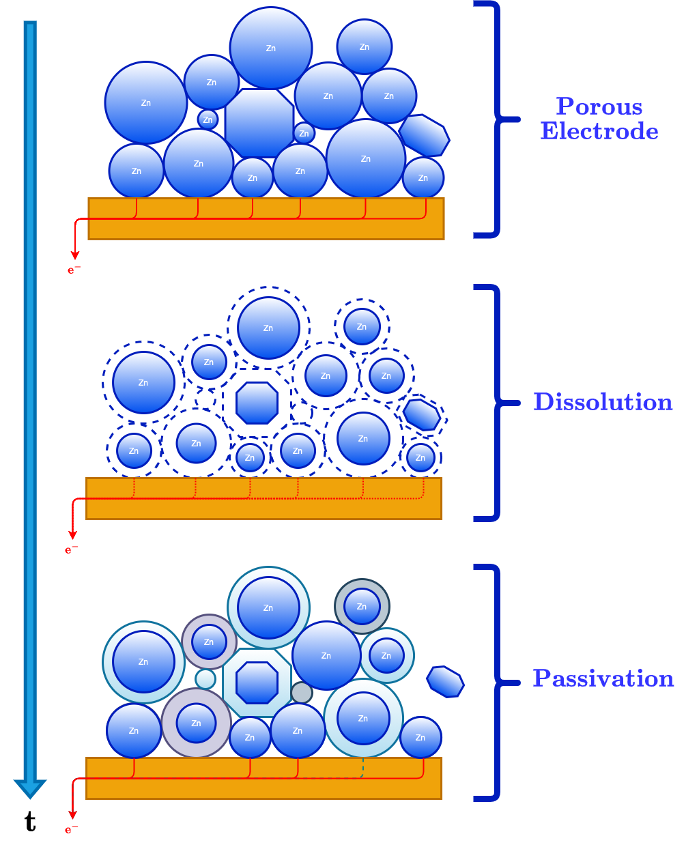
This paradigm is developed by considering one particle in a porous electrode system and adapting two different modeling approaches into a single framework using zinc as an example: (1) phase-field (PF) modeling and (2) reaction engineering. PF modeling has successfully been applied to moving boundary problems driven by thermal and electrochemical driving forces. For example, PF models can capture how the surface morphology evolves in problems regarding dendritic solidification during supercooling. Similarly, they have been extended to model dendritic growth during electrodeposition or plating. However, its diffuse description of the interface does not accommodate a description of the complex chemical kinetics at the electrode interface, such as those used in reaction engineering, which is not without its shortcomings. In reaction engineering, a surface site balance is used to model complex heterogeneous reaction kinetics, but this description faces an obstacle when applied to moving boundaries. Surface site balances are closed when the total number of surface sites is finite, which is essential to closing this balance for the analysis of heterogeneous reaction networks. This constraint is predicated on the Langmuir description of reaction kinetics, which assumes that the surface area of the interface is constant and, by extension, the total number of surface sites. Therefore, a modeling paradigm that combines key features of both modeling approaches is put forth to better understand porous electrode systems, such as the zinc anode.
This work aims to (1) identify key limitations in traditional porous electrode theory and modeling approaches and (2) introduce an amended framework that merges concepts from interfacial science, reaction engineering, and transport phenomena. The analytical tools used to study mass transfer and reaction kinetics can be applied from this amended framework. For example, the rate of passivation and electrodeposition as a function of surface curvature, temperature, etc., can be compared at the surface of the anode. Meanwhile, dendrite formation in electrodeposition, attributed to be a mass transfer limited process, can be examined in the same modeling framework. There are two networks of interest that we gain insight into: (1) the connectivity of particles in the porous electrode and (2) the set of complex coupled reaction kinetics (electrochemical and otherwise). Thus, reconciling these two modeling methodologies provides a deeper insight into porous electrode systems using zinc or other metal-electrode materials in general.