2025 AIChE Annual Meeting
(405b) Platinum and Gold Supported on Transition Metal Nitrides for Hydrogen Evolution in an Alkaline Electrolyte
Authors
Xue Han, Virginia Tech
Sinwoo Kang, Brookhaven National Laboratory
Hanjun Zhao, Columbia University
Shyam Kattel, Florida A&M University
Jingguang G. Chen, University of Delaware
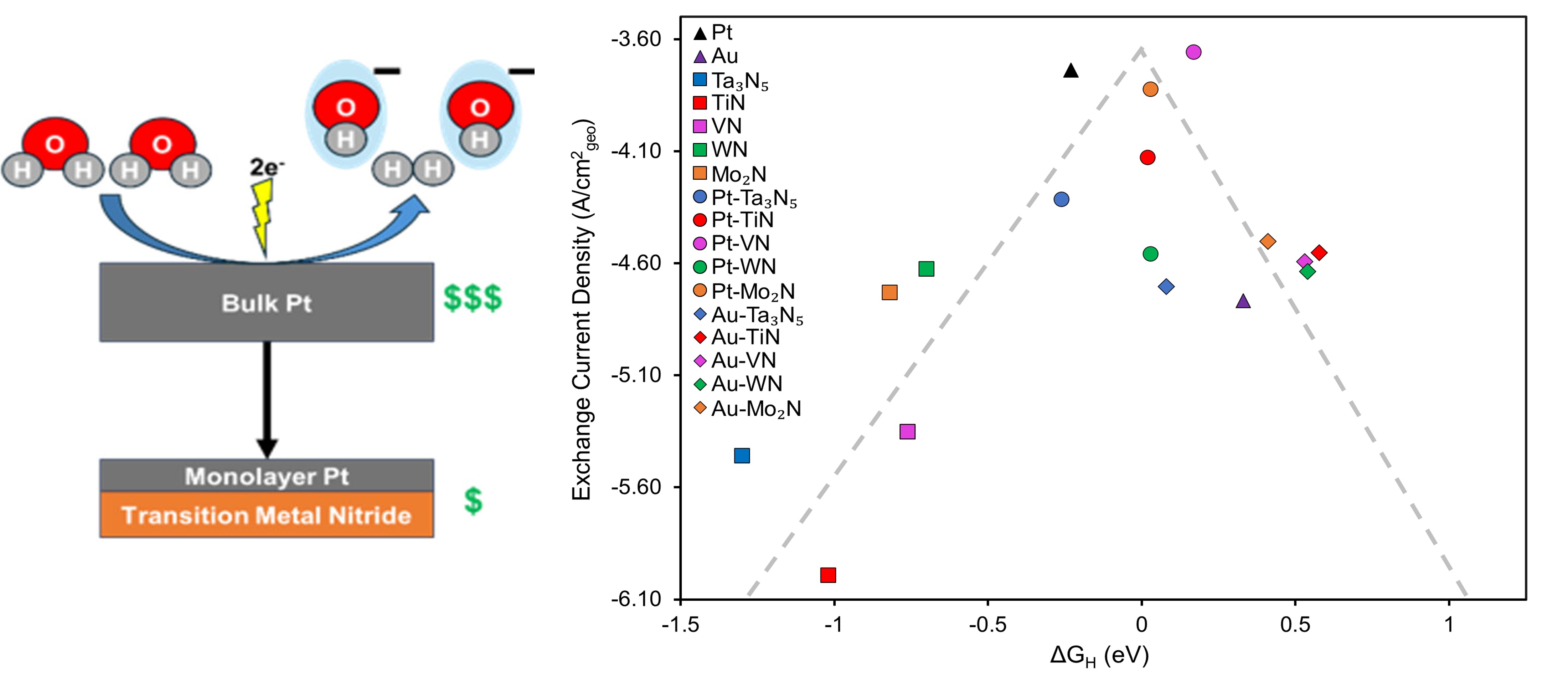
As the urgency to reduce reliance on fossil fuels increases due to carbon dioxide emissions, hydrogen produced by renewably powered water electrolysis has emerged as a promising technology. Alkaline electrolyzers typically exhibit lower current densities than acidic electrolyzers due to the slow kinetics of the hydrogen evolution reaction (HER) under alkaline conditions. This work developed Pt- and Au-modified transition metal nitride (TMN) thin films for improving alkaline HER kinetics. One monolayer Pt–VN, Pt–Mo2N, and Pt–TiN were the most promising thin-film catalysts, with alkaline HER activity approaching that of a bulk Pt foil. Additionally, the Gibbs free energy of adsorbed hydrogen was identified as a useful descriptor for alkaline HER activity on TMN and TMN-supported catalysts and has the potential to guide future studies on TMN-based catalysts for enhancing alkaline HER. For practical applications, the thin-film catalysts were then extended to Pt- and Au-modified TMN powders for alkaline HER. Both 5 wt % Pt/TiN and 2 wt % Pt/TiN powders exhibited lower overpotentials at 5 mA/cm2 when normalized by the Pt electrochemical surface area than the commercial 5 wt % Pt/C benchmark, suggesting a Pt–TiN synergy that creates opportunities for more cost-effective alkaline HER cathodes. Moreover, 20 wt % Au/Mo2N also displayed an enhancement in HER activity when compared to the commercial 20 wt % Au/C benchmark.