2025 AIChE Annual Meeting
(502e) Piston Reactor for Blue Hydrogen Production through Gas-Phase Steam Methane Reforming and Autothermal Reforming Routes
Authors
- Introduction
The implementation of electrified and compact reformers represents a promising approach to intensify hydrogen production from natural gas and biomethane feedstocks while minimizing carbon emissions[1]. Electrification is essential for integrating and storing intermittent renewable energy sources in the hydrogen production process, enabling sustainable, modular, and distributed production. This approach is particularly beneficial in regions with limited gas reserves and in capturing stranded gas that would otherwise be flared[2].
Piston reactor technology introduces an innovative approach for converting electrical energy into mechanical energy and subsequently into chemical products[3, 4]. This is achieved by utilizing rapid adiabatic compression-expansion cycles, which allow the reactor to generate high-temperature and pressure pulses in just milliseconds. The fast gas expansion effectively quenches the reacting mixture, preventing secondary reactions of metastable species that could result in unwanted by-products. This study evaluates the potential of gas-phase steam methane reforming (SMR) and autothermal reforming (ATR) as hydrogen production methods in the piston reactor, conducting a theoretical assessment to explore the reactor’s unique operating conditions, limitations, and potential benefits. A zero-dimensional, single-zone thermodynamic model was developed for a four-stroke, single-cylinder piston reactor to predict the temporal evolution of temperature, pressure, and chemical species, using a gas-phase mechanistic model[5].
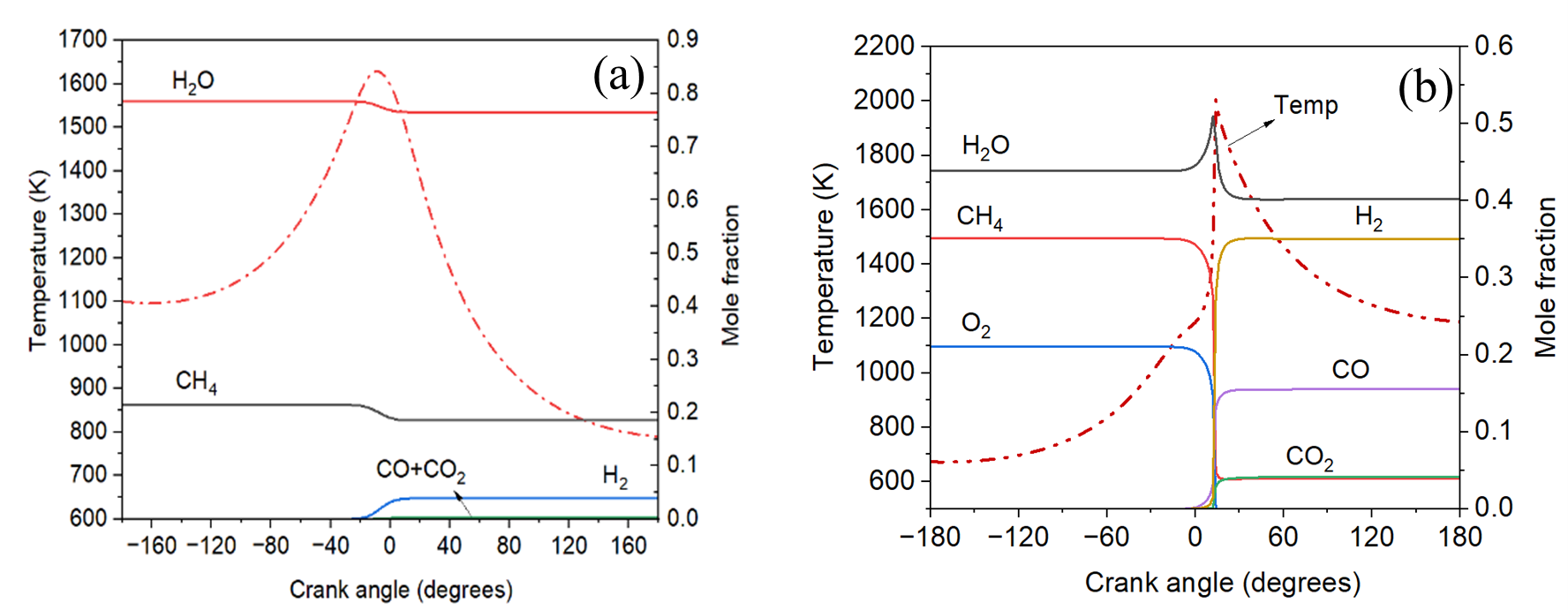
Figure 1(a) shows the baseline scenario for a 400-cc piston reactor operating under SMR conditions. In this case, an intake pressure of 1 bar and an H2O/CH4 ratio of 3.56 were applied. The methane conversion reached only 12%, and 4 mol% hydrogen was produced, with an intake temperature of 1100 K, the minimum required to initiate the reaction. Reducing the operational speed increased the residence time, which could improve conversion, but the associated heat losses at low speeds led to a decrease in both conversion and hydrogen production, falling below the baseline. The introduction of argon into the feed mixture was also explored to enhance in-cylinder temperatures by reducing the mixture's heat capacity. It was determined that a 60 mol% argon dilution was necessary to achieve methane conversions above 80%. As a result, the highly endothermic SMR reaction proved impractical under reasonable operating conditions and was excluded from further evaluations. In contrast, ATR showed promising results for hydrogen production, as depicted in Figure 1(b). This method used an H2O/CH4 ratio of 1.25, an O2/CH4 ratio of 0.6, and a lower intake temperature of 673 K compared to the SMR case. Under these conditions, the piston reactor achieved 89% methane conversion and produced 35 mol% hydrogen, similar to industrial catalytic processes, operating in the gas phase without a catalyst. When compared to a simulated industrial packed-bed ATR reactor, the piston reactor’s hydrogen production rate was significantly higher, reaching 3545 kg H2/m³·hr, compared to the industrial reformer’s 1702 kg H2/m³·hr. This highlights the piston reactor's advantage for modularized hydrogen production. A process flowsheet was developed integrating the piston reactor for ATR-based hydrogen production. Economic and emissions analysis showed favorable economics even at a small production scale of 25 tons/day of hydrogen, with CO2 emissions comparable to large-scale industrial ATR systems. The piston reactor's poly generation capability also enables the production of excess power and heat, which can be used for heat and power integration within the process, meeting energy demands for carbon capture and resulting in the production of blue hydrogen.
Figure 1. Evolution of species and in-cylinder temperature as a function of crank angle for
(a) case of SMR at N = 3000 RPM, T_intake = 1100 K, P_intake = 1 bar, H2O/CH4 = 3.561,
(b) case of ATR at N = 3000 RPM, T_intake = 673 K, P_intake = 1 bar, H2O/CH4 = 0.75, O2/CH4 = 0.6
References
- Oni, A.O., et al., Comparative assessment of blue hydrogen from steam methane reforming, autothermal reforming, and natural gas decomposition technologies for natural gas-producing regions. Energy Conversion and Management, 2022. 254: p. 115245.
- Anderson, D.M., et al., Sorption-Enhanced Variable-Volume Batch–Membrane Steam Methane Reforming at Low Temperature: Experimental Demonstration and Kinetic Modeling. Industrial & Engineering Chemistry Research, 2015. 54(34): p. 8422-8436.
- Abousrafa, A., et al., Piston reactor for chemical energy storage: Modeling study to explore electro-mechanical conversion route using propane feedstock. Chemical Engineering and Processing - Process Intensification, 2024. 202: p. 109840.
- Ashok, A., et al., Review of piston reactors for the production of chemicals. Reviews in Chemical Engineering, 2023. 39(1): p. 1-30.
- Abousrafa, A., et al., Model-based evaluation of piston reactor to produce hydrogen from methane via gas-phase SMR and ATR routes. Energy Conversion and Management, 2024. 321: p. 119036.
Acknowledgments. This work was made possible by funding from Qatar National Research Fund (QNRF) project number NPRP12S-0304-190222 and co-funding by Qatar Shell Research and Technology Center (QSRTC). The statements made herein are solely the responsibility of the author(s).