2025 AIChE Annual Meeting
(135e) Phosphatase-Excluding Polymer Micropatches for Enhancing Cytotoxic T-Lymphocytes-Based Cancer Therapy
Authors
Vinny Chandran Suja, Stanford University
Kolade Adebowale, Harvard University
Yu Xing Teo, Harvard University
Michael Griffith Bibbey, Harvard University
Leah Lourenco, Harvard University
Bolu Ilelaboye, Harvard University
Tatsuya Fukuta, Wakayama Medical University
Danika Rodrigues, Harvard University
Kyung Soo Park, Harvard University
Samir Mitragotri, Harvard University
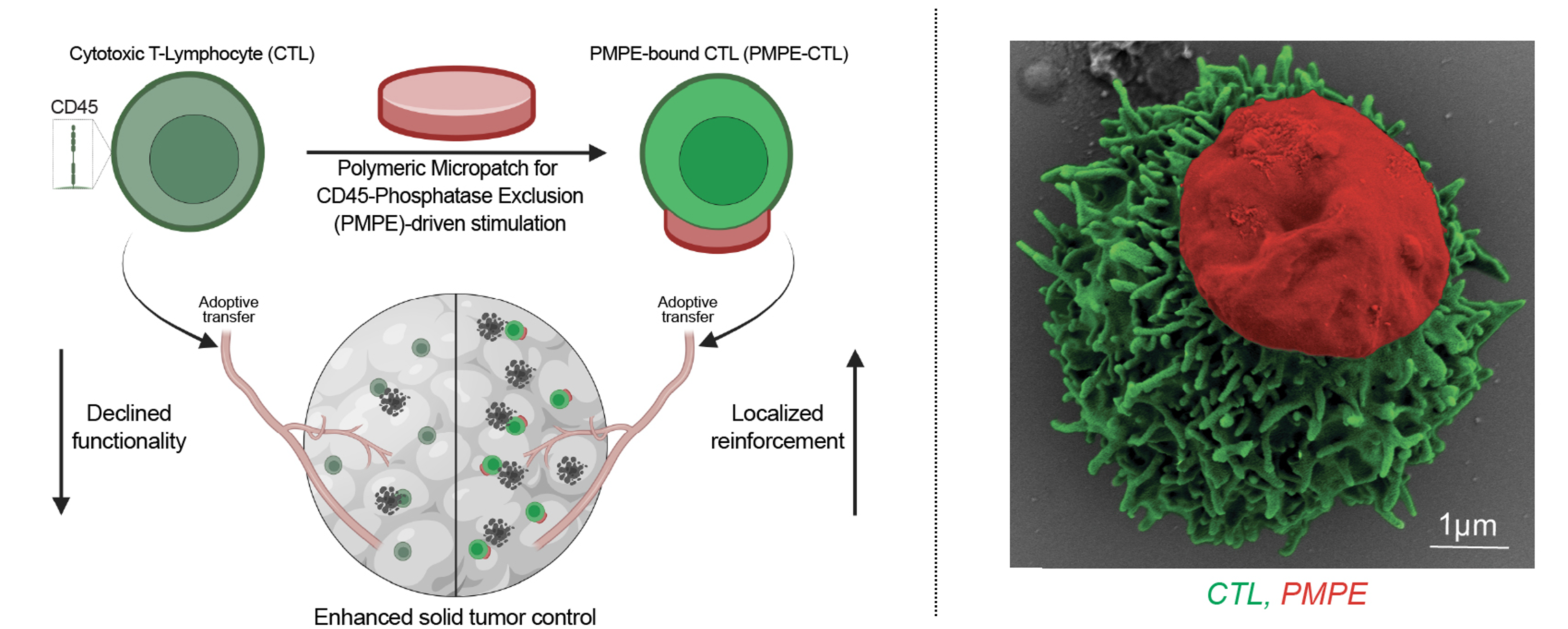
Despite decades of progress in cytotoxic T lymphocyte (CTL) therapies, their potential in solid tumors falls short of the remarkable success of CAR T cells in hematological malignancies. A major hurdle in solid tumors is their challenging microenvironment, which hinders CTL accessibility and renders adoptively transferred CTLs hypofunctional before they reach their targets. Recent studies show a rapid decline of CTL effector function within hours of infusion, even in CTLs primed ex vivo to have robust effector and proliferation capacity. Thus, engineering strategies that sustain CTL functionality post-transfer are of interest. Here, we present a cell engineering platform using Polymeric Micropatches for CD45-Phosphatase Exclusion (PMPEs), a biomaterial-based approach that modulates CTL phenotype and sustains their functionality post-infusion. Fabricated from polylactic-co-glycolide (PLGA) biopolymer, PMPEs are stable under physiological conditions over two weeks. PMPEs bind efficiently to CTLs and remain stable under physiologically relevant conditions, including shear stress and freeze-thaw cycle. Upon binding to CTLs, PMPEs induce micron-scale exclusion of CD45-phosphatase at the contact interface. This exclusion, validated through diffusion-signaling-based mathematical modeling and experimental imaging, provides an effective biochemical mechanism for tonic CTL stimulation. PMPE modification induces transcriptomic changes, as revealed by bulk RNA-seq, that enhance CTL effector potential. A comprehensive set of in vitro assays, including ELISA, calcium flux, immunofluorescence, proliferation, and cytotoxicity alongside in vivo studies such as biodistribution, immunophenotyping, and pathology analysis, demonstrate that PMPE-CTLs exhibit enhanced persistence, a robust type 1 immuno-permissive response, and excellent tolerability. In aggressive B16F10 melanoma models, PMPE-CTLs significantly improved tumor control, with 50% survival beyond 25 days, compared to none in the unmodified CTL group. When combined with a systemic IL15 superagonist (IL15sa) cytokine therapy, PMPE-CTLs prolong 46% survival beyond 35 days, with 15.4% complete tumor remission, compared to none in the IL15sa + CTL group alone. Altogether, PMPEs represent a simple but effective biomaterial-based approach for modulating CTL behavior, sustaining function post-transfer, and enhancing therapeutic efficacy in solid tumors. Their ease of use and scalability offer new opportunities for seamless integration into clinical CTL manufacturing workflows, advancing solid tumor management.