2025 AIChE Annual Meeting
(52g) Phase-Dependent Promoting Effect of Surface Oxygen on Molybdenum Carbide Catalysts during Formic Acid Electrooxidation
Authors
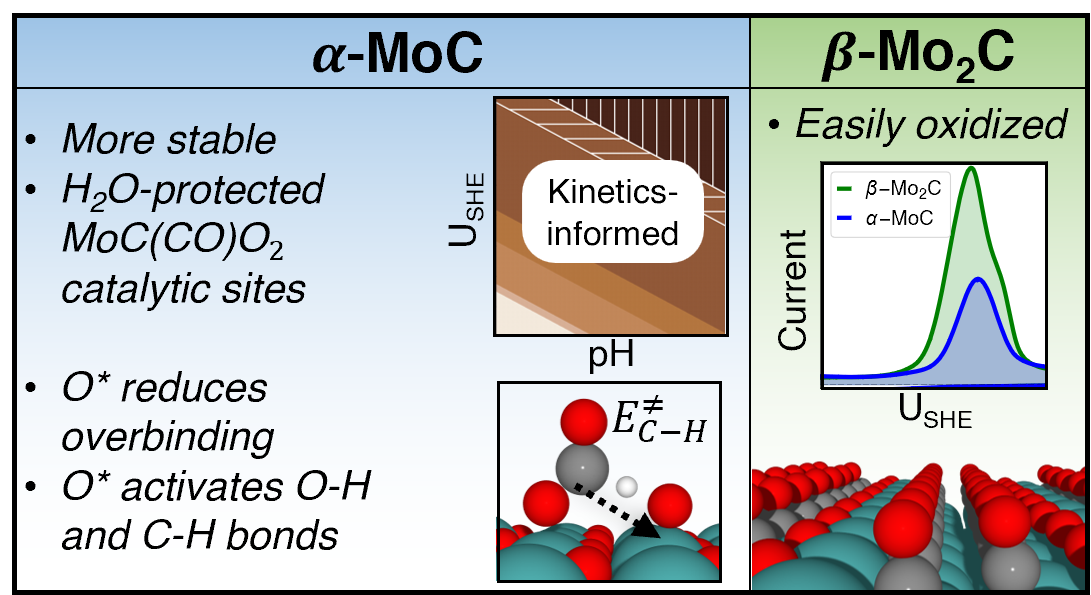
Here, we elucidate the surface structure and catalytic activity of α-MoC and β-Mo2C phases under electrochemical conditions using density functional theory and kinetics-informed ab initio thermodynamics. Both the surfaces were found to be extensively oxidized with the α-MoC phase showing greater stability, consistent with experimental CV predictions. Our oxidation model accurately predicts the extent of charge transfer associated with surface oxidation and also provides a mechanistic explanation for the surface species observed in XPS.
The effect of the resulting surface oxidation was found to be detrimental towards HER [1] on the β-Mo2C(011)-C since all metal sites O*/OH* covered. In contrast, partial surface oxidation on the α-MoC-(311)-Mo has a promoting effect towards the formic acid electrooxidation [2], as the surface oxygen weakens otherwise strongly bound intermediates. The α-MoC exposes H2O-protected [MoC2O2] and [MoC(CO)O2] oxycarbidic motifs available for catalysis in a wide potential window. It shows Pt-like O*-mediated O-H and C-H bond activation pathway. The α-MoC hosts active sites such as O*/Motop which are, however, blocked by H2O and HCOOH yielding no electrooxidation current. Pt doping was investigated computationally making the surface less oxophilic, suggesting possible design strategy towards active and selective carbide electrocatalyst.
[1] Yu et al. J. Mater. Chem. A, 2024, doi: 10.1039/D4TA01746C
[2] Gautam et al. ACS Catalysis, 2025, accepted doi: 10.1021/acscatal.4c06544