2025 AIChE Annual Meeting
(39g) pH Controlled Selective Precipitation of CaCO3 and MgCO3 from Mine Tailings: A Population Balance Modeling Approach
Authors
As the atmospheric CO2 concentration continues to rise, innovative approaches to carbon capture and storage (CCS) are crucial. One such strategy gaining traction is carbon mineralization, which offers a stable and permanent form of CO2 sequestration by converting it into solid carbonate minerals such as calcium carbonate (CaCO3) and magnesium carbonate (MgCO3). Beyond its environmental impact, the process holds strong economic potential since these carbonates have wide applicability in agriculture, construction, paper, and pharmaceutical industries. The abundant availability of these metals (Ca and Mg) in industrial waste streams, mine tailings, and seawater sources further enhances the viability of this approach [1].
By employing mine wastes for indirect carbon capture, high-purity carbonates (CaCO3 and MgCO3) can be produced [2]. Based on the composition of the waste material, the critically extracted ions (Ca2+ and Mg2+) can be selectively precipitated using atmospheric CO2. However, the fluctuating material composition of the waste materials in the feed, poses a significant challenge, since it introduces uncertainty in both the efficiency and selectivity of the precipitation process. The variability affects not only the phase purity and morphology of the final products but also the particle size distribution. To address the feed fluctuations, this study focuses on the controlled selective precipitation of the carbonates from multi-ion solution, supported by a robust population balance modeling (PBM) framework.
Methodology
In this study, the development of a precipitation model for the sequential precipitation of CaCO3 and MgCO3 using alkaline-rich waste material solutions and the experimental validation of the model for the pH-based process control strategies will be presented. The model integrates thermodynamic equilibrium parameters with kinetic equations for nucleation and crystal growth, allowing dynamic simulation of the precipitation process under various pH control strategies [3]. The key component of the approach is a population balance model (PBM) that tracks the evolution of particle size distribution (PSD) over time. The PBM includes both primary nucleation and crystal growth, with rates driven by local supersaturation levels resulting from CO2 dosing and pH regulation. This enables not only the estimation of the carbonate yield, but also facilitate the control of the particle size and morphology and to steer the process towards a state of the maximum productivity, maintaining desired purities for the carbonate products.
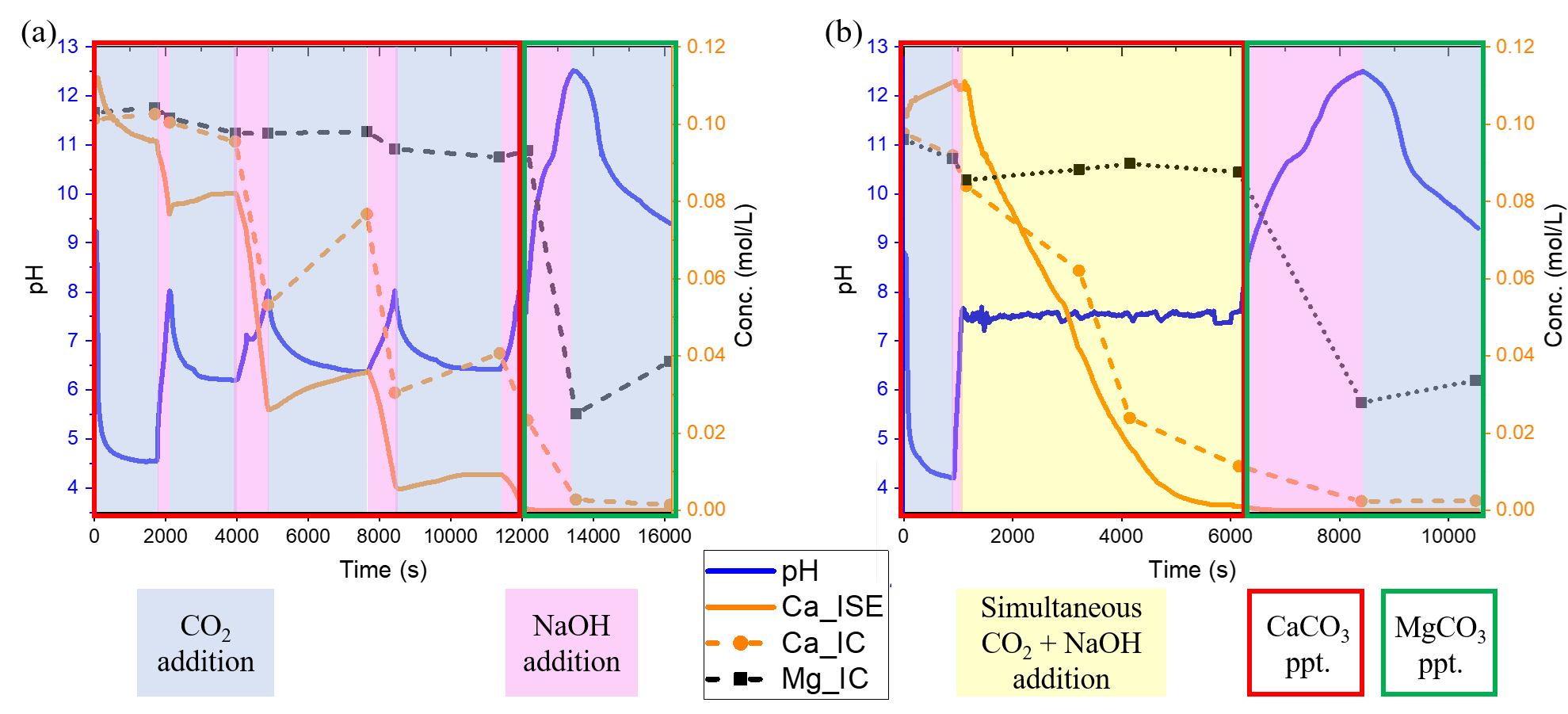
To support model development and validation, a semi-batch precipitation setup was employed, chosen for its inherent flexibility and controllability of the precipitation process due to its ability to provide gradual and precise addition of reactants. This approach ensures uniform mixing and prevents localized supersaturation, which could result in undesired by-products or inconsistent crystal properties [4]. The key factors influencing the process include pH, ion concentration, and mixing conditions. In this setup, CaCO3 and MgCO3 were precipitated sequentially by exploiting the pH-dependent solubility differences between the two species. Precise pH control, achieved through alternating CO2 and NaOH dosing, was essential for avoiding co-precipitation and ensuring product quality. Depending on the dosing scheme two pH control strategy implementation: pH swing and pH constant, has been presented in Figure 1. Both methods prove effective for the precipitation of CaCO3 and MgCO3 sequentially, one after the other, from multi-ion solutions. The two strategies, however, differ in nucleation and growth dynamics, leading to distinct particle morphologies and PSDs, which are captured and analysed through the PBM framework. Depending on the target particle properties, either strategy can be adapted to meet specific application requirements.
Results and Discussion
To evaluate the effectiveness of the pH control strategies and validate the process model, experiments were conducted using both the pH control approaches. The influence of key parameters such as pH, CO2 partial pressure (PCO2), and seed particles were analyzed using real-time PSD monitoring alongside offline characterization techniques including ion chromatography (IC), X-ray diffraction (XRD), and scanning electron microscopy (SEM) [3], [5]. The experimental results revealed distinct precipitation dynamics for each pH control strategy. While the pH swing strategy promoted rapid supersaturation spontaneous nucleation of CaCO3, followed by MgCO3 formation as the pH is increased. In contrast, the pH constant approach offered a more controlled crystal growth following nucleation, resulting in bi-modal PSDs. These distinct behaviors highlighted the influence of pH regulation.
These experiments provided the necessary data for the formulation and validation of the first precipitation process model. A semi-batch process model was implemented to simulate the time evolution of both, liquid-phase ion concentrations and solid-phase particle characteristics. The coupled model included population balance equations for each carbonate phase, with kinetic expressions fitted to experimental data. The PBM framework was modelled using the method of moments which the model to track key characteristics of the particle population, without resolving the full PSD, offering a computationally efficient and accurate precipitation model. The model was used to study the impact of the pH control trajectory on the resulting PSD under variation of the final processing time and to derive an optimal pH control strategy [6].
Keywords: Selective precipitation, pH control, population balance modeling, mine tailings
References
[1] E. R. Bobicki, Q. Liu, Z. Xu, and H. Zeng, “Carbon capture and storage using alkaline industrial wastes,” Apr. 2012. doi: 10.1016/j.pecs.2011.11.002.
[2] A. A. Olajire, “A review of mineral carbonation technology in sequestration of CO2,” 2013, Elsevier B.V. doi: 10.1016/j.petrol.2013.03.013.
[3] C. Hegde, A. Voigt, and K. Sundmacher, “Control strategies for the selective precipitation of CaCO3 from multi-ion solutions,” Mar. 31, 2025. doi: 10.26434/chemrxiv-2025-vqllg.
[4] C. Steyer, M. Mangold, and K. Sundmacher, “Modeling of particle size distribution for semibatch precipitation of barium sulfate using different activity coefficient models,” Ind Eng Chem Res, vol. 49, no. 5, pp. 2456–2468, Mar. 2010, doi: 10.1021/IE901306R.
[5] C. Hegde, A. Voigt, and K. Sundmacher, “Towards pH Swing-based CO2 Mineralization by Calcium Carbonate Precipitation: Modeling and Experimental Analysis,” Computer Aided Chemical Engineering, vol. 53, pp. 1519–1524, Jan. 2024, doi: 10.1016/B978-0-443-28824-1.50254-4.
[6] N. Bajcinca, B. Heydaryan Manesh, M. Al Khatib, M. Babazadeh, and K. Sundmacher, “Closed-Form Solutions for Control of Calcium Carbonate Precipitation,” Apr. 01, 2025. doi: 10.26434/chemrxiv-2025-hg5dt.