2025 AIChE Annual Meeting
(439h) Particle and Porosity Characterization of Dry Battery Electrode Powders
Authors
With the increased demand for lithium ion batteries (LIBs), there is a strong push toward reducing production costs, improving electrochemical performance, and adopting more sustainable manufacturing practices. One of the major advancements in this field is the transition from conventional slurry-based electrode coating methods to dry coating techniques. Dry coating eliminates the need for hazardous solvents, reducing environmental impact while also offering potential enhancements in battery performance and manufacturing efficiency.
A key component in the dry coating process is the fibrillation of polytetrafluoroethylene (PTFE) binders, which serves as a binder within the electrode composite. When mixed with active materials and conductive additives, PTFE forms an interconnected network that ensures mechanical integrity and electrical conductivity. To optimize the dry coating process and improve battery performance, it is critical to characterize the physical properties of the electrode/PTFE mixture and establish correlations between material properties, fabrication parameters, and final application performance.
In this study, both unfibrillated premixes with 1−5 % PTFE and fibrillated samples produced from these premixes with different process parameters were systematically examined. Characterization techniques included gas pycnometry for skeletal density, gas adsorption for BET surface area, mercury intrusion porosimetry for pore size distribution and total pore volume, porosity, and dynamic image analysis for particle size and morphology. Both the premixes and the fibrillated samples were investigated to understand the impact of PTFE content and the mixing time on the surface characteristics and material performance.
The results reveal clear differences in material properties as a function of binder content and mixing time, highlighting their influence on electrode structure and processability. These insights contribute to the development of optimized processing strategies for next-generation LIB manufacturing, supporting the transition toward greener and more efficient battery production technologies.
Results and Discussion
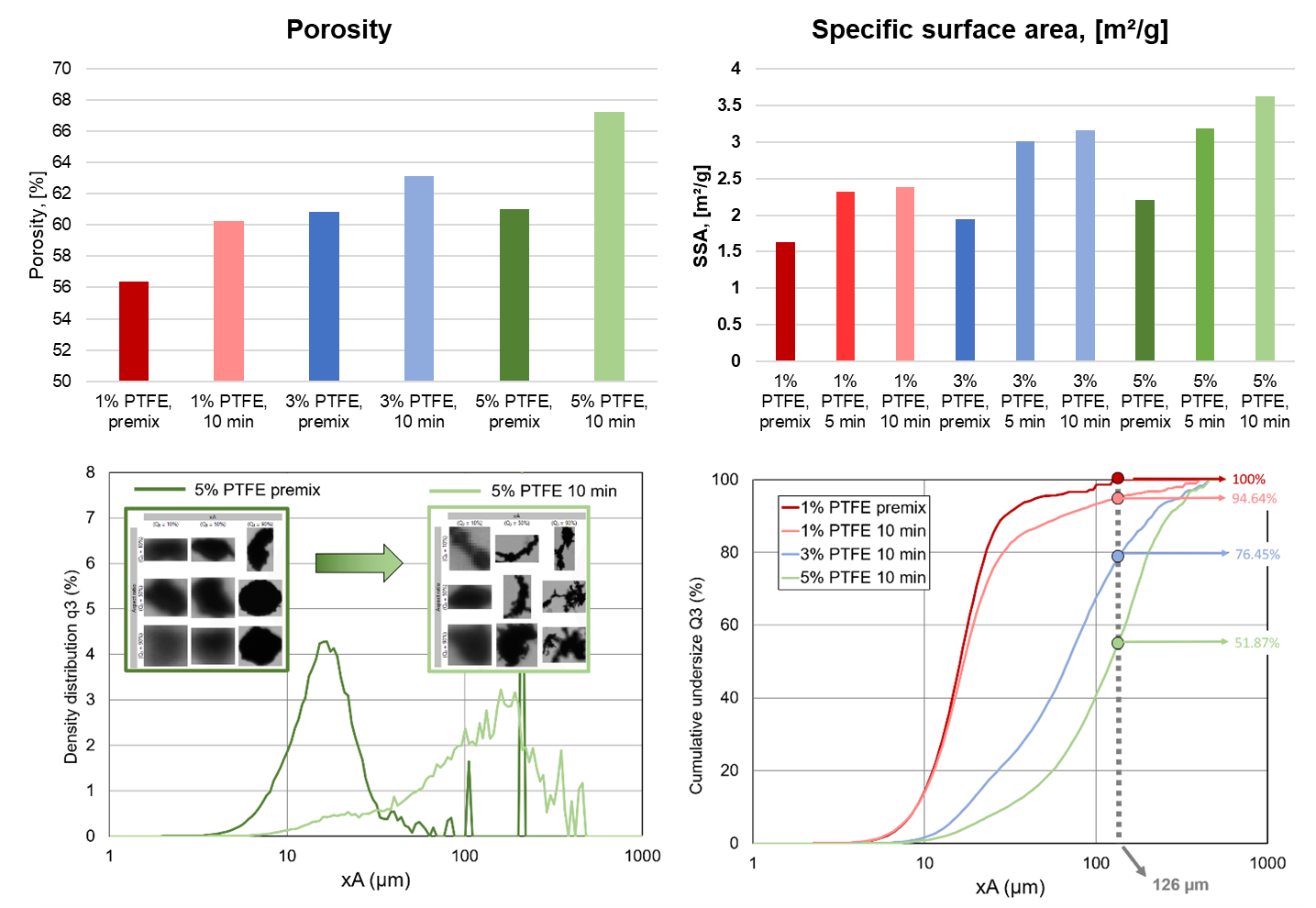
Porosity of the prepared powder mixtures was determined using a combination of gas pycnometry to determine the skeletal volume and mercury intrusion porosimetry to determine the pore volume. Because pure PTFE and graphite are expected to have similar skeletal densities, the skeletal density of all samples was found to be essentially the same, regardless of PTFE content. However, mercury porosimetry reveals different pore volume for the materials, where the trend is increasing pore volume with increasing PTFE content. Percent porosity is calculated from these two values and a trend of increasing porosity with increasing PTFE content is also observed (see Figure). Pore size distributions were also obtained from the mercury intrusion data and show the distribution of the processed samples shifts to slightly smaller pore size from the premixes.
N2 (77 K) BET surface area was measured on the three premixes and samples processed for different times. It was found that surface area increased with increasing PTFE content and that surface area also increased from the premixes to processed samples as shown in the Figure.
Dynamic image analysis (DIA) was performed to assess the particle size and shape distributions of various samples. Prior to measurement, samples were pre-dispersed in 15 mL of isopropanol with three drops of Triton X-100 added as a surfactant to improve wetting and minimize agglomeration. To effectively capture fibrillated particles formed after mixing, both 1× and 6× magnifications were used, enabling high-fidelity imaging of both fine and coarse structures.
Particle size was quantified using the projected area equivalent diameter (xA) - the diameter of a circle with the same area as the particle’s 2D projection. Shape analysis was based on circularity, a parameter ranging from 0 (irregular) to 1 (perfect circle), calculated from the relationship between the particle’s perimeter and area. This metric sensitively reflects particle elongation, roughness, or fibrillation.
These measurements enabled detailed comparisons between sample conditions before and after mixing (example for 5% PTFE shown in the Figure). Fibrillation fraction was defined as the proportion of particles with a volume-based xA greater than 126 µm. An increase in PTFE content corresponded to a higher fibrillation fraction, with the trend showing a strong fit to a quadratic function. Furthermore, higher PTFE content was also associated with a decrease in circularity, indicating the formation of more irregular fibrillated structures. The higher fibrillation factor and decrease in circularity observed for increasing PTFE content also agrees well with the BET surface area and porosity trends, with the fibrillation contributing to both.
Conclusion
The characterization techniques shown here provide valuable insight into the particle and porosity characteristics of these dry battery electrode powders and how they are affected by processing of the material. Correlations are seen between the amount of PTFE in the sample and the percent porosity and BET surface area, which may be attributed to both the degree of PTFE fibrillation and the smaller particle sizes of the processed samples. These physical properties can be used to guide the initial raw mixture components and the processing time in order to optimize the final electrode.
Acknowledgements
We thank Ahmed Elabd at The Chemours Company for providing the samples.