2025 AIChE Annual Meeting
(487a) Partial Reprogramming By Cyclic Nanog Overexpression Reverses Senescence, Restores Metabolism and Improves the Function of Skeletal Muscle in Aged Mice
Authors
Shahryar Shahini - Presenter, University at Buffalo
Fred Earl, University at Buffalo
Sai Harsha, University at Buffalo
Yali Zhang, University at Buffalo
Kirkwood Personius, University at Buffalo
Stelios Andreadis, State Univ of New York-Buffalo
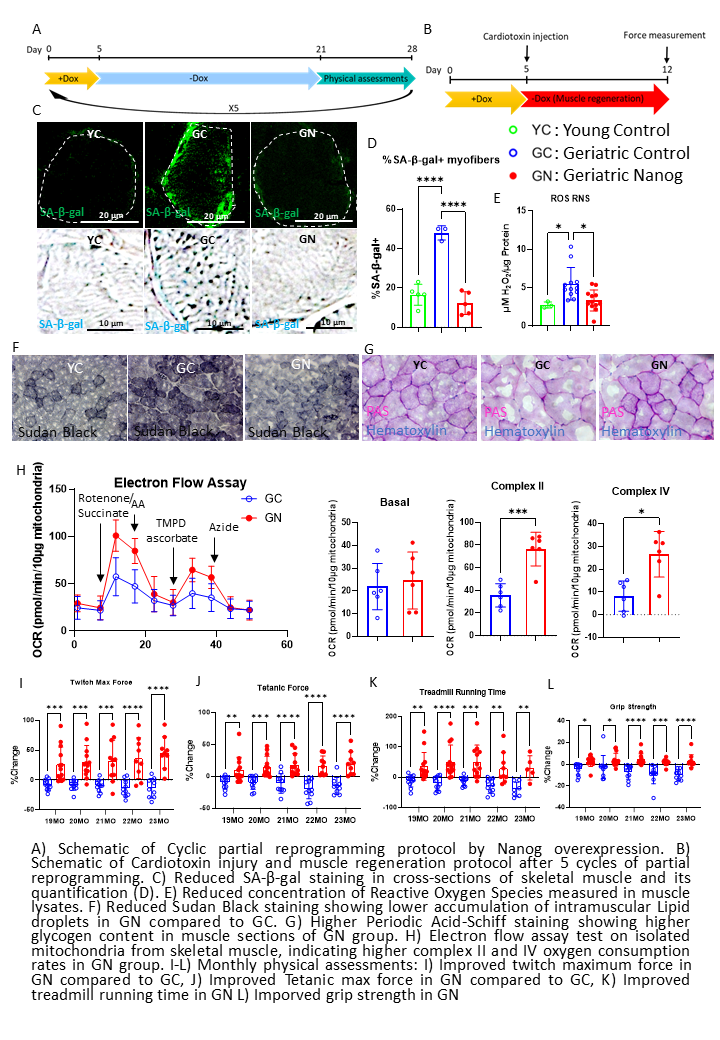
Sarcopenia or age-related decline in muscle mass, strength, and regeneration limits mobility and quality of life in the elderly. A key driving force behind sarcopenia is impaired muscle regeneration following stem cell dysregulation, cellular senescence, and metabolic deterioration. Recent evidence underscores the potential of cellular reprogramming in mitigating age-associated cellular and tissue decline. Several studies from our laboratory reported that local expression of the key pluripotency transcription factor, Nanog can induce partial reprogramming, restoring the hallmarks of aging and enhancing muscle regeneration after injury in naturally aged mice. Here we explore whether systemic and cyclic Nanog administration can reverse aging and restore muscle function and regeneration capacity in naturally aged mice. Aged mice (18–24 months old) were treated with monthly cycles of doxycycline-induced Nanog overexpression for 5 days per cycle. Muscle function was evaluated through in vivo measurements of twitch and tetanic force, as well as grip strength and uphill treadmill performance. After 5 cycles, animals were subjected to another 5 days of doxycycline treatment and tissues were isolated after one week of its withdrawal. Senescence markers, including SA-β-gal activity, p21 expression, and reactive oxygen species (ROS), were assessed using fluorogenic staining and immunofluorescence. Furthermore, RNA sequencing was conducted on RNA isolated from the tibialis anterior muscle to investigate transcriptomic changes induced by Nanog overexpression. Finally, Mitochondrial function was analyzed via electron flow assay to evaluate the activity of respiratory chain complexes II and IV. To assess lipid accumulation, Sudan Black staining was performed on skeletal muscle cross-sections. Our results showed that cyclic Nanog expression over a period of five months from age 19 to 24 mo old mice significantly reduced several senescence hallmarks, including SA-β-gal activity, the fraction of p21+ nuclei and levels of reactive oxygen species (ROS) in muscle. RNA-seq and GSEA analysis revealed significant upregulation of biological processes involved in ribosomal biogenesis, DNA repair, RNA processing, and transcriptional activation, indicating improved cellular maintenance and protein turnover. Sudan Black staining showed increased lipid droplet accumulation in aged muscle that was reversed by cyclic administration of Nanog. Similarly, PAS staining showed that Nanog increased glycogen levels that were abnormally low in aged muscle. Finally, measurements of mitochondrial function using Seahorse metabolic analysis showed that Nanog preserved the activity of complex II and IV, as shown by increased oxygen consumption rates in electron flow assay. Most notably, physical assessment tests showed that Nanog-treated mice exhibited improved grip strength and endurance as evidenced by increased running distance and time in an uphill treadmill test. Finally, direct measurements of the forces exerted by the dorsiflexor muscles upon electrical stimulation showed that Nanog increased muscle strength that declined over time in naturally aged control mice. Taken together, our findings demonstrate that Nanog-induced partial reprogramming reverses molecular, metabolic, and functional features of aging in skeletal muscle without reprogramming to the pluripotent state.