2025 AIChE Annual Meeting
(120b) Parametric Optimization of Wireless ?led-Packed Bed Reactor Enabling for Continuous Photochemical Transformations
Authors
Esai Daniel Lopez - Presenter, Worcester Polytechnic Institute
Andrew Teixeira, Worcester Polytechnic Institute
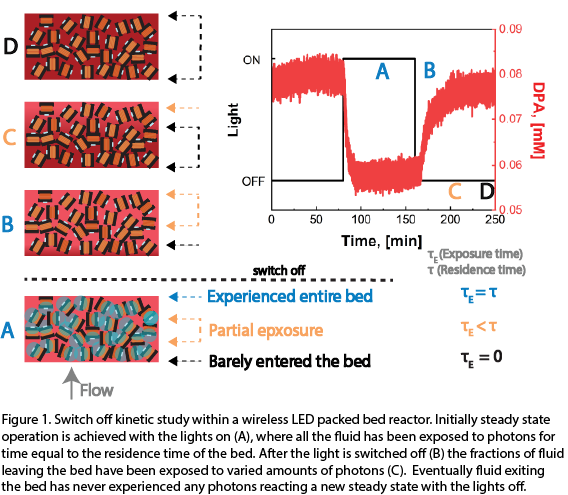
During the design of flow photochemical reactors a balance must be achieved between the photons supplied to the system, mixing time of the reactants, and reaction time. Poor mixing and too small of a photon dosage will result in concentration and illumination gradients, with overall slower kinetics. Longer residence times are then required to observe substantial conversion, rendering the reaction unfit for continuous flow manufacturing. To unlock operation in the optimal regime a new geometry of reactor was created. 250 wireless µLED lights were packed within a quartz column and illuminated using an induction field external to the column, creating a µLED PBR. This configuration has several advantages compared to traditional PFR and batch photoreactors. Namely, a PBR suffers two orders of magnitude less pressure drop than PFR of the same submillimeter characteristic length. Photons are supplied directly to the reactants creating a homogenously illuminated environment. The lights, act as passive mixers creating turbulence ensuring fresh reactant is constant passing over the photon source. In this work a novel transient in-situ “switch off” kinetic study was performed with a known actinometry reaction, the oxidation of 1,9-diphenylanthracene (DPA) with the photocatalyst tris(2,2′-bipyridyl)ruthenium(II) chloride (Ru(bpy)3Cl2). In doing so the conversion was measured for a continuum of photon exposure times as well as catalyst and reactant concentrations. Increasing the light intensity of the bulbs, by employing a stronger induction field resulted in enhanced reaction kinetics. By varying the catalyst loading, reactant concentration, and light intensity the operating regime was tuned to optimize the reactor’s performance.