2025 AIChE Annual Meeting
(182at) Paper-Based Microfluidic Device for Blood Plasma Multi-Nutrient Assay By Colorimetry: Towards Resource-Efficient Disease Diagnostics
Authors
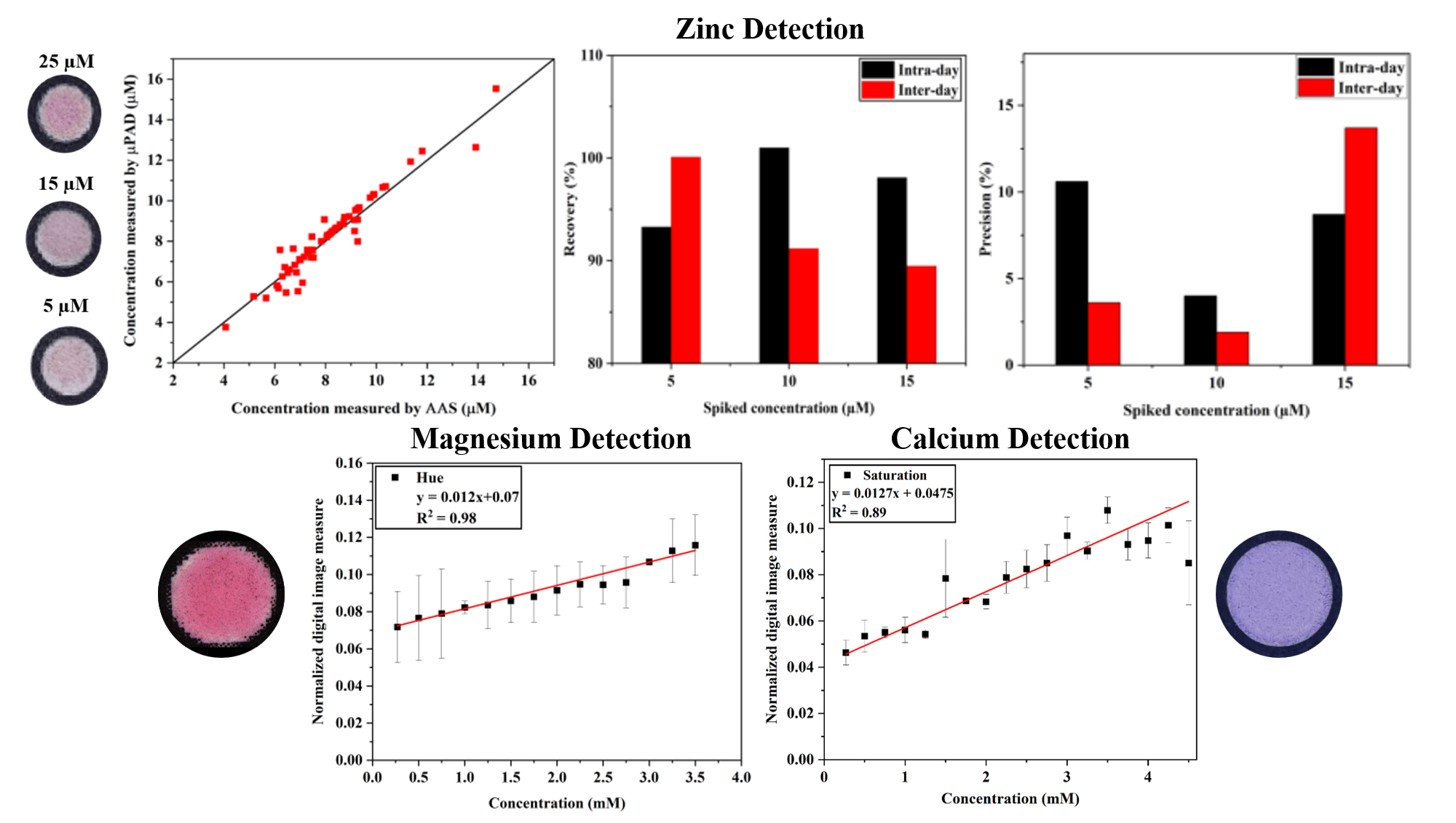
This study involves the fabrication of a paper-based microfluidic device for the quantitative determination of zinc, magnesium and calcium in blood plasma for different physiological levels. The hydrophobic barriers needed for these devices are fabricated using commercial desktop printers. The images of the reaction zones are captured using a mobile phone camera and the intensity of the digitized image are correlated to the concentrations. We have also designed an automated image analysis application program in the MATLAB platform.
For Zinc detection, an average absolute error of 5.06% suggests the potential of the µPAD in blood plasma zinc assay with a resolution of 2 µM to differentiate between the healthy and zinc deficient population groups. Sensitivity and specificity of the device are found to be 95% and 76%, respectively. Moreover, the method is also found to be 97% accurate (intraday) with 8% repeatable (intraday). The MATLAB application is found to be repeatable and reliable (with ±6.5% color intensity difference). We have also obtained calibration plot for magnesium assay with R2 = 0.98 and for calcium assay, the coefficient of linearity for the calibration plot was obtained to be R2 = 0.89 that can be used for analysis of real blood plasma samples (ongoing work).
A multiplexed device for combined micronutrient assay for the detection of zinc, calcium, and magnesium altogether is to be fabricated. The design and fabrication of this multiplexed µPAD incorporates essential principles of chemical engineering, including transport in porous media, microfluidic flow regulation, colorimetric reaction engineering, and automated data processing, rendering it highly pertinent for chemical engineers specializing in healthcare technologies. The method is straightforward, rapid, cost-effective, and does not require any complex equipment or fabrication protocol. Thus, its potential in point-of-care testing is worthy of further clinical trial.