2025 AIChE Annual Meeting
(274c) Paper-Based Biased Alternating Current Electrophoresis Enables Rapid, Tunable Biomolecule Enrichment for Point-of-Care Diagnostics
Author
Paper-based diagnostic platforms such as lateral flow assays (LFA) and vertical flow assays (VFA) are widely adopted for point-of-care (POC) testing due to their low cost, simplicity, and rapid turnaround. Despite their clinical utility across various diseases, these assays often suffer from poor sensitivity. This limitation primarily arises from two factors: they depend on passive sample transport (e.g., capillary flow), which prevents analyte enrichment, and. A key unmet need in advancing POC molecular and proteomic diagnostics is the ability to enrich target analytes prior to detection. To address this, we previously discovered a novel electrokinetic mechanism, termed biased-alternating current electrophoresis (b-ACEP), or paper based-biased ACEP (pb-ACEP), which enables directional migration of charged species toward regions of higher electric field density without inducing chemical reactions. Here, we hypothesize that b-ACEP can be translated onto porous media to enable active enrichment directly on paper-based devices. We demonstrate that screen-printed carbon electrodes on cellulose substrates can generate tunable electric field gradients, allowing rapid and controllable enrichment of biomolecules. These results validate b-ACEP as a promising mechanism for enhancing sensitivity in next-generation POC assays.
Materials and Methods
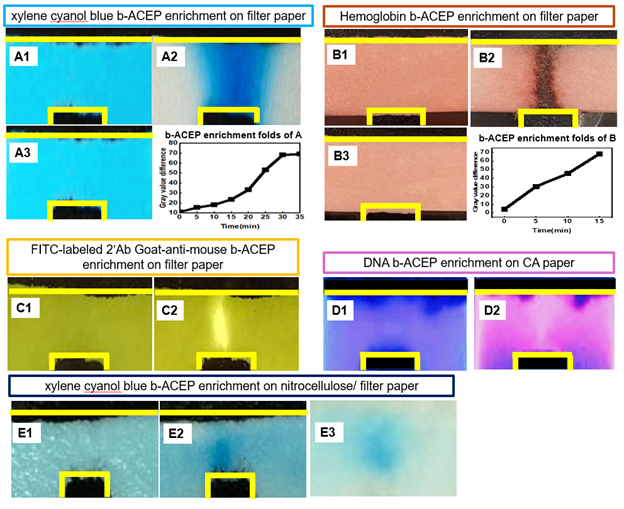
To establish the versatility of the paper-based b-ACEP (pb-ACEP) platform across diverse paper substrates and biomolecular targets, we systematically evaluated its performance on three commonly used porous media: filter paper (pore size = 25 µm), cellulose acetate paper (pore size= 0.45 µm), and nitrocellulose paper (pore size=0.2 µm). The platform operates by generating spatially non-uniform electric fields to drive directional migration of charged species via b-ACEP. For filter paper and cellulose acetate, carbon electrodes were patterned via screen printing, while nitrocellulose paper—due to its delicate structure—employed carbon tape electrodes. In all cases, electrodes were positioned orthogonally with a 5 mm gap to establish a well-defined field gradient. To demonstrate broad molecular applicability, we tested enrichment of multiple analytes including 1,000 bp DNA, hemoglobin, FITC-labeled immunoglobulin G (IgG), and xylene cyanol blue dye. A sinusoidal voltage was applied using a waveform generator and commercially available amplifier. Image acquisition was performed using a standard smartphone camera, and the spatial enrichment profiles were quantified using ImageJ and MATLAB. This workflow illustrates a robust, low-cost, and modular approach for integrating b-ACEP enrichment onto various paper-based diagnostic formats, highlighting its potential for enhancing sensitivity across a broad range of biomolecular assays.
Results and Discussion
We report the development of a pb-ACEP platform for active biomolecule enrichment on porous substrates, aiming to enhance the sensitivity of paper-based point-of-care diagnostics. The system was tested across three widely used paper materials—filter paper (pore size=25 μm), cellulose acetate (pore size= 0.45 μm), and nitrocellulose (pore size= 0.2 μm)—to evaluate material-dependent performance. Carbon electrodes were screen-printed or affixed with conductive tape to create spatially non-uniform electric fields, and sinusoidal voltages (10 V peak-to-peak, 50–100 Hz) were applied. The pb-ACEP platform enabled consistent enrichment of xylene cyanol blue (Figure. A), hemoglobin (Figure. B), FITC-labeled IgG (Figure. C), and DNA (Figure. D) across different substrates. Notably, xylene cyanol showed 10-fold enrichment on filter paper (Figure. A2&4), 5-fold on nitrocellulose (Figure. E1&E3), and 20-fold for DNA-GelRed on cellulose acetate (Figure. D1&2) under 10 V, 100 Hz. Hemoglobin achieved up to 100-fold enrichment at 10 V, 50 Hz on filter paper (Figure B2&4). Enrichment efficiency was strongly influenced by both material pore structure and electrophoretic properties of the analytes. Additionally, xylene cyanol enrichment increased with higher voltage and decreased with increasing frequency, demonstrating tunable performance. These results establish pb-ACEP as a generalizable, low-power, and reaction-free method for molecular enrichment on paper substrates, offering a promising strategy to improve the analytical sensitivity of next-generation paper-based assays.
Conclusions
We developed a novel pb-ACEP assay that leverages spatially biased AC electric fields to actively enrich biomolecules on paper-based substrates. By applying low-voltage sinusoidal signals through patterned carbon electrodes, we achieved rapid (<15 min) and tunable enrichment of diverse targets, with enrichment factors reaching up to 100-fold depending on molecular properties and substrate type. This reaction-free and low-cost method is broadly compatible with common porous materials. Given its generalizability and operational simplicity, pb-ACEP holds strong potential for integration into existing paper-based diagnostics such as LFAs and VFAs, significantly enhancing their sensitivity for point-of-care disease detection.