2025 AIChE Annual Meeting
(189z) Palladium-Platinum Nanosheets for Hydrogen Sensing
Authors
Sadaf Mohsenifard - Presenter, University at Buffalo (SUNY)
Thomas Thundat, University at Buffalo (SUNY)
Mark Swihart, University at Buffalo
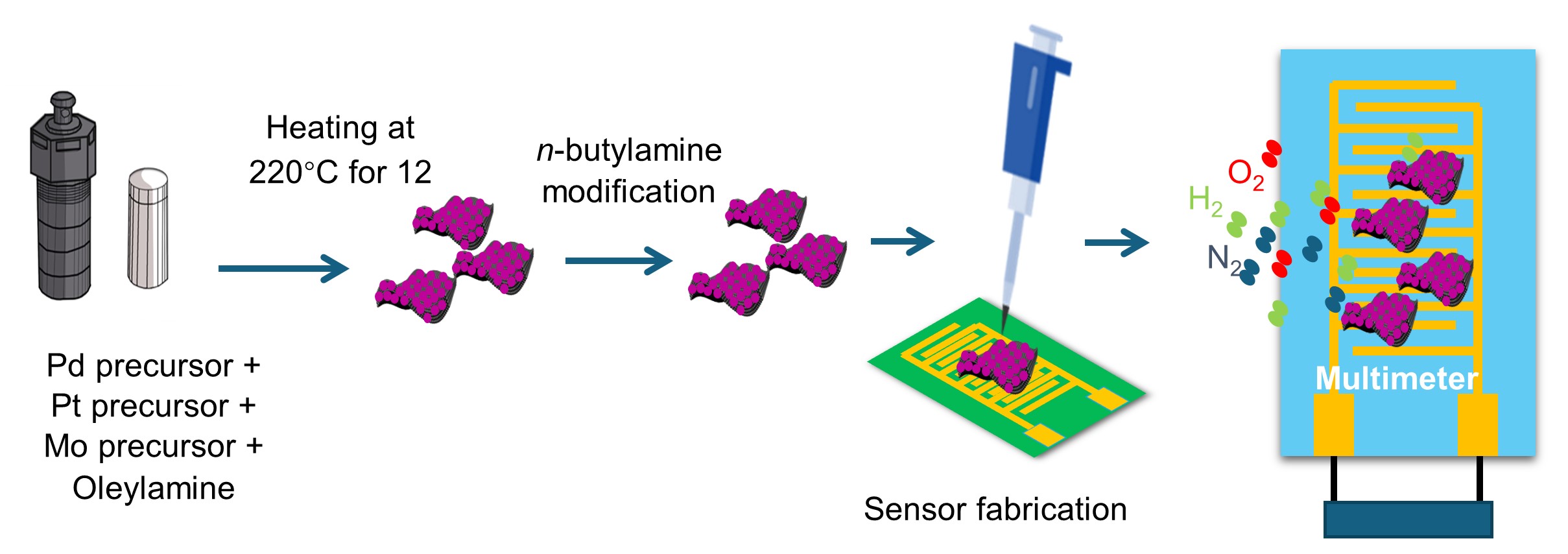
Hydrogen is being extensively investigated for use as a carbon-free energy carrier, especially in fuel cell vehicles and for long-term energy storage. Given that H2 can create explosive mixtures in air at concentrations as low as 4%, safe use of H2 at a consumer level will require inexpensive, low-power hydrogen sensors that can be widely deployed. Here, we used a new technique for making ribbon-like palladium-platinum nanosheets (PdPt NSs). We created sensors via drop-casting dispersions of these nanosheets onto interdigitated electrodes. In these chemoresistive sensors, the resistance increases in the presence of hydrogen, due to rapid uptake of hydrogen by the PdPt nanosheets at room temperature, forming a hydride with higher resistivity than the hydrogen-free nanosheet. The most promising PdPt nanosheet sensors, with 5:8 and 7:6 Pd:Pt ratios showed response times of 16 s and 14 s, and responses of 6.0% and 4.4%, respectively, to 1 vol% H2 in air. These sensors could readily measure as little as 100 ppm of H2 in air while maintaining positive response (increasing resistance upon exposure to H2). These sensors have potential to broadly support the safe use of H2 as an energy carrier because they function at ambient temperature.