2025 AIChE Annual Meeting
(406h) Oxidation State Modulation Via Surface Restructuring: The Impact of Fe Incorporation into Ni Catalyst on Activity and Stability in AEM Water Electrolysis
Authors
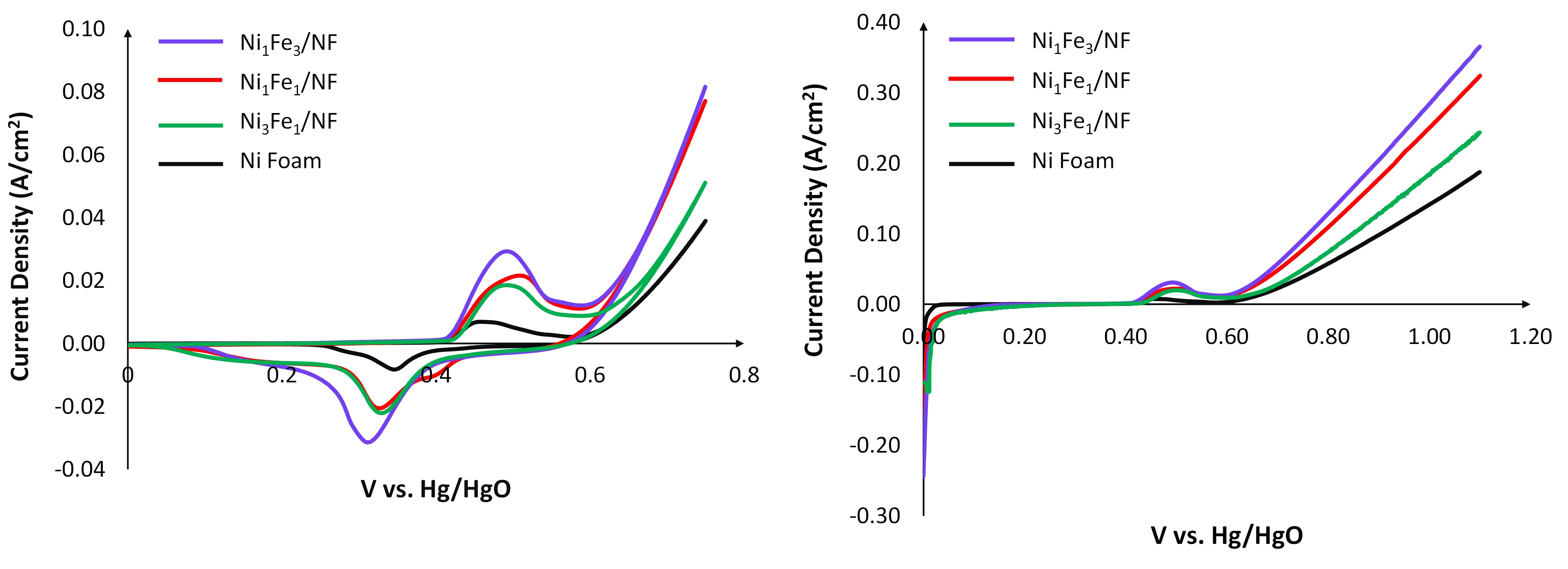
Here, we synthesized and tested a series of Ni:Fe nanoparticles supported on Ni Foam (NF), with corresponding metal ratios of Ni3Fe, Ni3Fe2, NiFe, and NiFe3 in both a 3-electrode cell and a continuous-flow AEM electrolyzer to better understand the role of Fe in modulating the OER rates. Our results indicate that Ni3Fe/NF and NiFe3/NF exhibit 1.33 and 2.09 times higher current densities, respectively compared to pristine NF. However, NiFe/NF and NiFe3/NF do not demonstrate significant differences in current density; suggesting the existence of an optimal Fe content in Ni/NF, beyond which further addition of Fe may no longer enhance current density. Our working hypothesis is that exceeding this threshold iron content might result in Fe atoms reducing the adjacent Ni atoms coordinated with oxygen. Such changes would increase the proportion of Ni2+ relative to Ni3+ within the structure acting as insulated sites, as Ni3+ is more electrically conductive than Ni2+. Consequently, this could lead to a drop in overall electronic conductivity.
Alternatively, there may be an upper Ni:Fe ratio above which Fe atoms are destabilized, resulting in Fe leaching and a concomitant increase in impurities into the electrolyte. A quantitative understanding of these behaviors, specifically the change in oxidation state of Ni from Ni3+ to Ni2+ while compensating for the difference in electronic charge by another element, could provide insights into the factors controlling activity and stability in AEMWE.