2025 AIChE Annual Meeting
(449g) Osmosis-Driven 3D-Printed Pump for Local and Targeted Chemotherapy in Glioblastoma
Authors
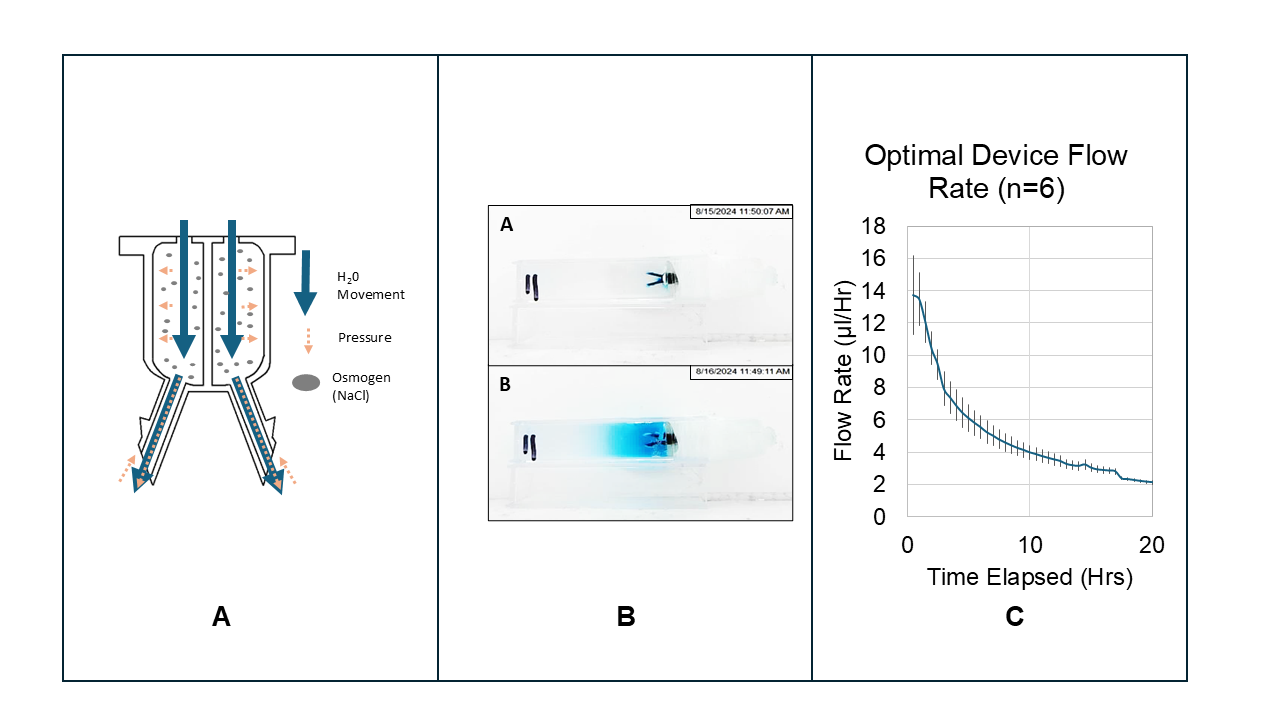
Glioblastoma is a malignant tumor that develops in the brain's white matter and typically measures around 4cm in diameter. This condition has a 5% five-year survival rate due to high recurrence rates despite surgical and chemotherapeutic interventions. The tumor propagates into healthy brain tissue, making it challenging to remove entirely without causing significant damage to the brain. The blood-brain barrier (BBB) makes systemic delivery of effective chemotherapy difficult, which allows residual cancer cells to continue growing. Recently, locally drug-delivering brain implants have been employed to bypass the BBB and deliver chemotherapy directly to the affected areas in the brain. While catheters and devices exist for localized drug delivery to the brain, they often suffer from occlusion, reflux due to high intracranial pressure (ICP), and functional failure. Additionally, most are not fully intracranial. The exception is the Gliadel Wafer, which is designed for implantation in the tumor resection cavity following surgery. This wafer reduces tumor growth and boosts survival rates by 37%. However, cauterized tissue at the excision site hampers drug diffusion. To address the above limitations of current practices, we created a 4mm diameter, osmotically driven, Acrylonitrile-Butadiene-Styrene (ABS) -like thermoset MicroFine™ resin 3D-printed brain implant with integrated needles that bypass the cauterized tissue to target lingering cancer cells directly. High postoperative ICP can hinder an implanted device's drug delivery and cause reflux. However, using osmotic pressure, the device can expel the drug until the reservoir is empty (Fig. A).
Methods and Results
We wanted to control the device delivery rate through the pump's design, so we chose four variables for investigation: needle length (2mm-4mm), diffusion opening diameter (50µm-500µm), membrane pore size (15nm-25nm), and osmogen concentration (0%wt-25.3%wt). These variables were chosen for their relationship with water entry, which was hypothesized to control the device's delivery rate. A design of experiments (DOE) approach with 12 experiments was developed with JMP software to determine these variable's impacts on delivery rate and diffusion distance. The target delivery rate was 3-5 µl/Hr since this rate was estimated to prevent total drug washout from the target area. A target distance of 25 mm was chosen because recurrence occurs within a 20 mm radius of the original tumor.
The device's performance was investigated using Brilliant Blue FCF (FDCBLUE1) as a drug analog and sodium chloride (NaCl) as an osmogen. We constructed a white box with a contained light source to avoid data inconsistency due to changing light sources altering the visual results. We ran each experiment within this box in a randomized order. The device was placed in a mounting structure, and the needles were inserted into a transparent cuvette filled with 0.2% agarose gel. A 4K HD Dell camera recorded an image every thirty minutes for 20 hours as the device released its payload into the gel (Fig. B). These images were then optically analyzed to extract the flow rate and diffusion distance data by custom software.
In all cases, the pump initially delivered its payload rapidly from between 5 µl/Hr to 20 µl/Hr due to an unpredictable burst effect. After approximately one hour, the flow rate quickly dropped to between 0.5µl/Hr and 3µl/Hr, depending on the design variables of the device. We generated two models from this data: a predictive model for flow rate and a predictive model for diffusion distance. The predictive flow rate model indicates that the variable with the most significant impact was the osmogen concentration. The second most important variable is the pore size of the semi-permeable membrane mounted on the top of the device, with larger pores causing the device to deliver its payload more quickly due to allowing the increased rate of entry of water molecules. The diffusion distance model indicates that the variable with the most significant impact on diffusion distance is the membrane pore size, followed by the membrane diameter. Needle length did not increase the diffusion distance, which indicates that diffusion distance hinges on the rate of delivery rather than its delivery position.
To verify that the flow rate and diffusion distance models could provide useful device behavior predictions based on the input variables, a device with a membrane diameter of 100 µm, an osmogen concentration of 25.3%, a needle length of 3mm, and a membrane pore size of 25 nm was assembled and tested six times. According to the results of these trials, this pump had an average flow rate of 2.5 ± 0.10 µl/Hr (Fig.C) and an average diffusion distance of 15.5 ± 0.40 mm, both of which are within the expected range predicted by the two generated models which predicted the device would deliver between 2.26-3.61 µl/Hr, and diffuse between 15.23-19.89 mm from the device.
Significance
The pump performance was slightly varied, but the concentration gradient and flow rate calculated were consistent across all six experiments. The two models predict device performance within a flow rate between 0.5-3.6 µl/Hr and a diffusion distance between 11.2-19.8 mm from the device. They can guide pump design based on flow rates, generate 1D drug diffusion profiles, and predict payload delivery duration from the flow rate. We will use this information to improve the design further and boost its performance. Experiments with glioblastoma in rats have shown that these devices work in the mammalian brain, even with potential postoperative edema and ICP. This device will be modified for application in large animal models to assess its performance in the human brain. Future versions of this device aim to deliver drugs directly to glioblastoma excision sites, bypassing tissue that blocks chemotherapy diffusion. This could reduce recurrence, lessen systemic side effects, and enhance patient quality of life.
Figure A: The working principle of the osmotically propelled device, with arrows representing water movement into and out of the device and pressure gradients, both driven by an osmogen's presence. Figure B: An example of images of the device at 0Hrs and 20Hrs, which were used with image analysis to extract the flow rate and the diffusion distance. Figure C: The optimal device's calculated flow rate over time (n=6) shows the device initially delivered very quickly due to the burst effect and then became more stable over time as it slowed.