2025 AIChE Annual Meeting
(11f) Optimization Under Uncertainty for the Production of Tributyl Citrate through Simultaneous Acidification–Esterification Using Calcium Citrate
Authors
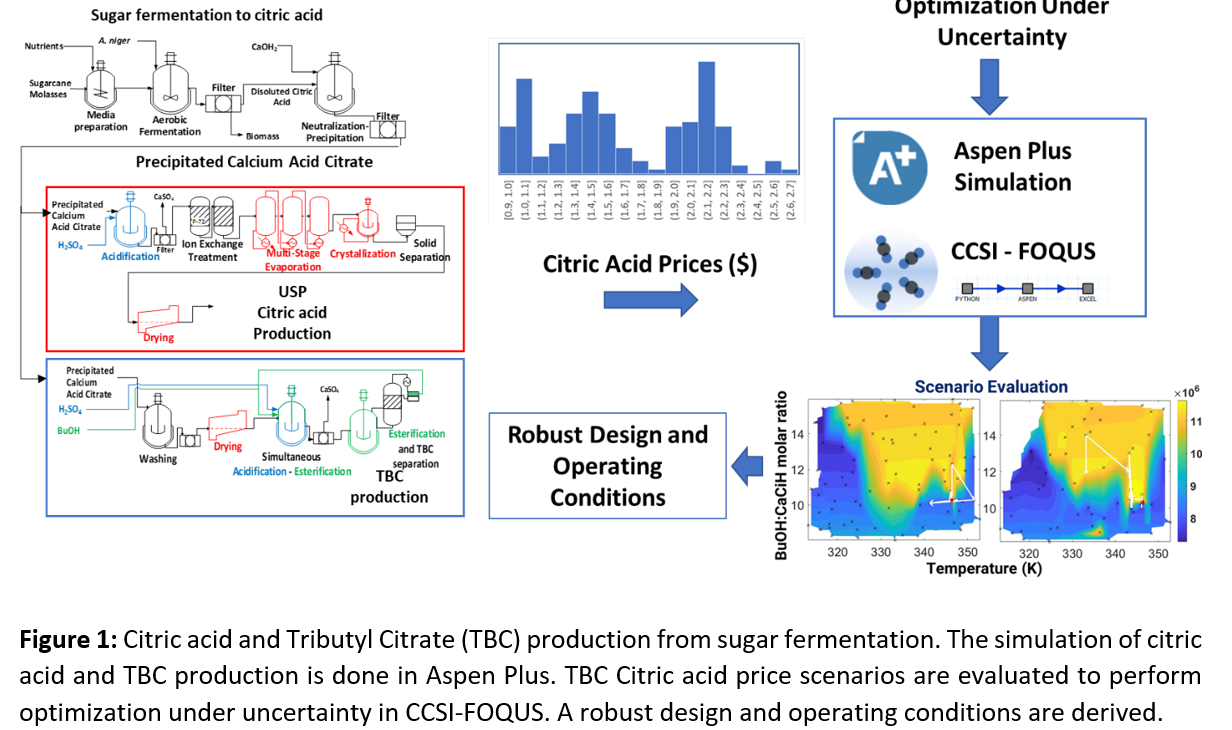
Traditional TBC production requires high-purity citric acid, which is obtained through submerged fermentation followed by multiple refining steps. Citric acid is generally recovered as calcium acid citrate via neutralization with lime, filtered, resuspended, and then acidified with sulfuric acid to release citric acid in water media, generating gypsum as a by-product. Then, water is removed using energy-intensive evaporation steps until crystal precipitate. Such crystals can be redisolved, recrystalized and dried until USP purity is achieved. From previous work, novel simultaneous acidification-esterification (SAE) was proposed and experimentally evaluated to sidestep most citric acid purification stages [1]. This process is carried out by mixing calcium citrate with sulfuric acid in a n-butanol solution, trigering the release of citric acid in the reactive medium where it is further esterified with the alcohol; here H2SO4 also serves as catalyst. In the experimental analysis, a kinetic model was proposed and it was found suitable for further process design and scale up. Then, by using the obtained model, this work focused on the simulation and optimization of the novel SAE process, comparing it to the traditional TBC production process. For the last, operating conditions employed in an industrial facility in Colombia were used.
In the industrial facility used as reference, only a minor fraction of produced citric acid is destined for TBC synthesis, and the same TBC production target were used to assess the SAE process. Additionally, it was envisioned that such process could be implemented in the existing facilities for TBC production, so the optimization was conducted considering operating costs [2].
Nevertheless, when using the SEE process involving acidification-esterification for TBC production, optimal operation and design must consider how large would be the fraction of citric acid that could be used to produce TBC in comparison to the used to produce high-purity citric acid. This decision is economically significant as citric acid prices are highly sensitive to international market fluctuations. When citric acid prices are high, its use as a raw material for TBC production may not be economically viable, making its recovery as a high-purity product the preferred option. Conversely, when prices are low, the cost-effectiveness of using citric acid for TBC production improves significantly, especially given TBC’s higher added value. Therefore, facility designs and operational strategies should be resilient and adaptable to such market variability.
To tackle this challenge, we implemented an optimization under uncertainty based on historical citric acid prices. From this distribution, representative price scenarios were selected, and detailed simulations were run using Aspen Plus® and Aspen Custom Models. The acidification-esterification kinetics were implemented as a user kinetic subroutine, and to perform the optimization under the evaluated scenarios, the Framework for Optimization and Quantification of Uncertainty and Surrogates (FOQUS) was used [3], allowing the integration of Aspen simulations with useful user-defined scripts (Python, Excel, etc.). This approach enabled the identification of optimal design and operating conditions that remain robust across varying market situations.
[1] A. F. Cabeza, D. E. Bernal Neira, and A. Orjuela 2025. Intensified Production of Butyl Citrates from a Calcium Citrate Salt via Solid-Liquid Reaction. Submitted. CEJ.
[2] A. F. Cabeza D. E. Bernal Neira, and A. Orjuela. 2024. A Novel Cost-Efficient Tributyl Citrate Production Process. Systems & Control Transactions 3, 121-128. doi: 10.69997/sct.122277
[3] D. C. Miller et al.2017. Innovative Computational Tools and Models for the Design, Optimization and Control of Carbon Capture Processes. In Process Systems and Materials for CO2 Capture, 1st ed., A. I. Papadopoulos and P. Seferlis, Eds., Wiley, 2017, pp. 311–342. doi: 10.1002/9781119106418.ch12.