2025 AIChE Annual Meeting
(503e) Optimization and Techno-Economic Analysis of Temperature Swing Adsorption Process for Post-Combustion CO2 Capture Using a Novel Adsorbent with Waste Heat Utilization
Authors
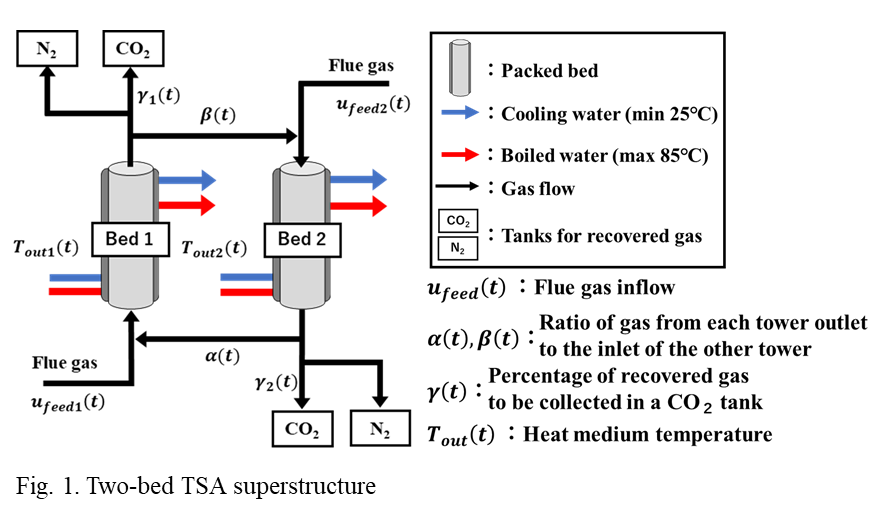
To solve this problem, we developed a novel PAU zeolite. This adsorbent shows a significantly higher CO2 working capacity than existing adsorbents at a low desorption temperature of 85°C [3]. Due to this feature, the PAU zeolite can be regenerated easily using waste heat. The isotherm measured experimentally was incorporated into a mathematical model of a two-bed TSA system. To identify the optimal design and operation, we employed a superstructure illustrated in Figure 1, which encompasses many different operating strategies including reflux operations [4]. Using this superstructure, the CO2 capture cost is minimized, evaluating the net present value considering both of the capital and operating costs of the TSA process. The optimization problem is solved by a deterministic solver to identify the optimal solution in a rigorous manner. This novel and systematic approach, which has not been reported in the past, allows us to fully explore the potential of the novel adsorbent. Furthermore, a sensitivity analysis was conducted to assess the impact of waste heat availability on CO2 capture costs.
References
[1] F. Nath, M.N. Mahmood, N. Yousuf, Recent advances in CCUS: a critical review on technologies, regulatory aspects and economics, Geoenergy Sci. Eng., 238 (2024), Article 212726,
[2] K.Z. House, C.F. Harvey, M.J. Aziz, D.P. Schrag, The energy penalty of post-combustion CO2 capture and storage and its implications for retrofitting the U.S. installed base, Energy Environ. Sci., 2 (2009), pp. 193-205,
[3] H. Lee, S. Hikima, R. Ohnishi, T. Takewaki, A. Katz, Privileged zeolitic sites for humid CO2 adsorption: K+ in double eight-membered rings, Chem. Commun., 60 (2024), pp. 10140-10143,
[4] Y. Sugiura, T. Yajima, Y. Kawajiri, Mapping adsorbent properties to optimal process performance of two-bed temperature swing adsorption by superstructure optimization, Chem. Eng. Process., 191 (2023), Article 109438,
Acknowledgment
This study was funded by New Energy and Industrial Technology Development Organization (Grant Number JPNP 20005).