2025 AIChE Annual Meeting
(390c) Optimal Hybrid Extraction–Distillation Process Design for Bio-Acetic Acid Recovery
Authors
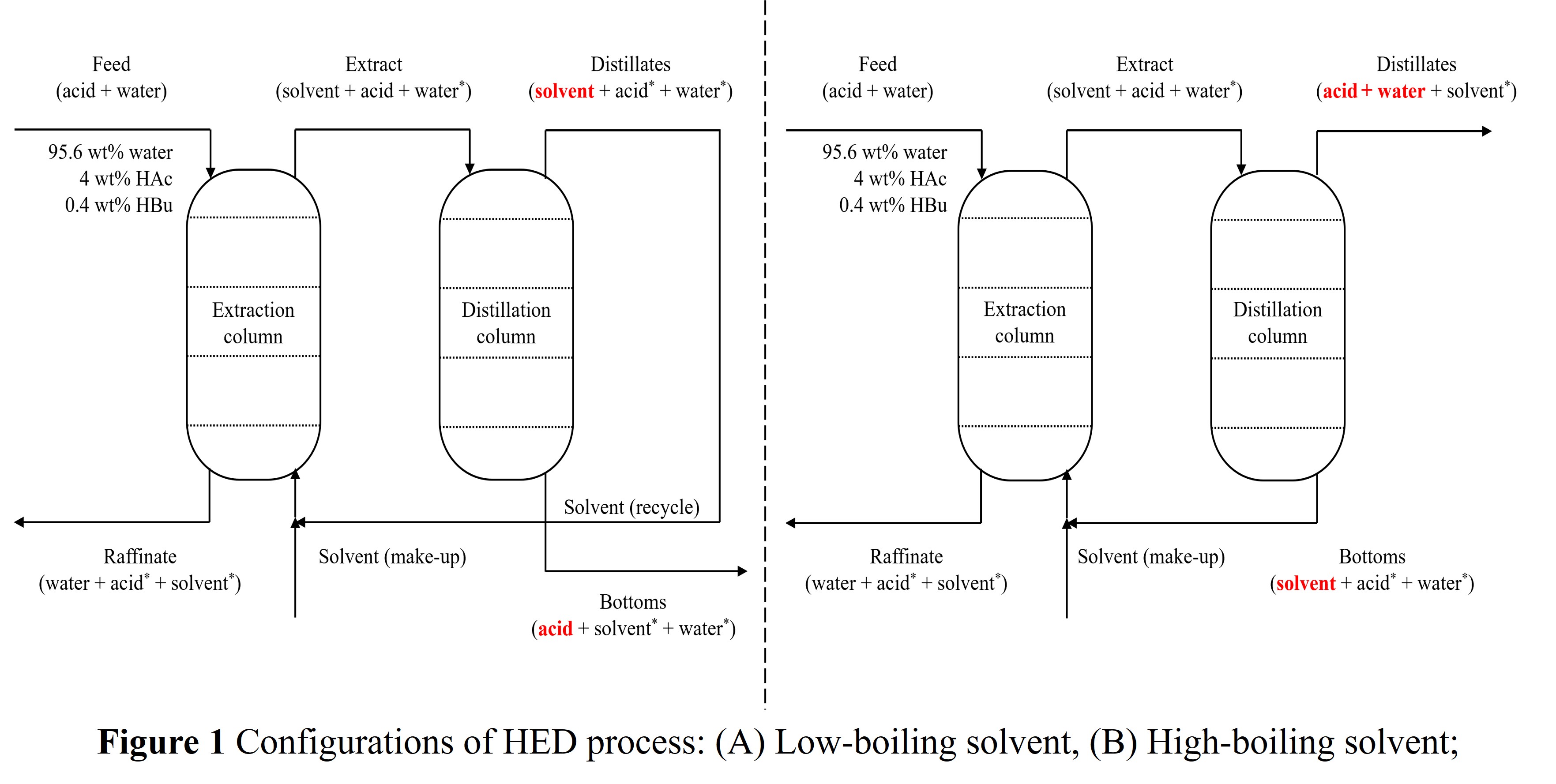
Process models were developed in Aspen Plus for both systems. To simulate the TOA-based process, we conducted LLE experiments and developed a Pitzer activity coefficient model, which was used to accurately predict extraction performance in the presence of carboxylic acids.
Key design variables, including solvent-to-feed ratio, reflux ratio, and distillation column configurations, were optimized to minimize separation cost (USD/kg-HAc). The TOA-based process demonstrated lower reboiler duty and solvent consumption, leading to improved energy efficiency. Despite TOA's higher price, its stronger extractive performance and reduced energy demand resulted in a competitive economic profile compared to the EA-based process.
This study highlights the critical role of solvent selection and thermodynamic modeling in designing cost- and energy-efficient downstream processes for bio-based HAc production, supporting the broader transition to low-carbon chemical manufacturing.