2025 AIChE Annual Meeting
(400h) One-Step Flow-Type Purification Process for Efficient Size-Selective Separation of Crude Organic Modified Nanoparticle
Authors
Organic modified nanoparticles (NPs) are metal oxide particles that have been surface-modified with organic compounds such as fatty acids. They are attracting attention as novel materials because they exhibit unique optical and electrical properties due to organic modification, and they also show excellent dispersibility in organic solvents1). To achieve these functions, it is essential to obtain NPs with uniform particle size2), and post-synthesis purification is required. Various separation methods have been proposed, but most of them have only been examined at the laboratory level3). Therefore, the conventional centrifugation separation method is used industrially. In this method, purification is performed by repeating batch operations multiple times, which makes the process complex and inefficient (60 g/month)4). This is a significant bottleneck in the practical application of NPs. For this reason, a highly practical and efficient purification method is strongly desired.
We propose a novel purification method for NPs using a flow-type column separation process with ion-exchange resin as a solid adsorbent. When gel-type anion-exchange resin was used, it was observed that all of injected NPs did not get retained and passed through the column, with the small, well-dispersed NPs flowing out first and the larger, aggregated NPs flowing out much later5). This behavior is completely opposite to that of conventional size exclusion chromatography, and is thought to be due to a new separation mechanism. In this study, to elucidate the mechanism of size-selective separation in this new method, we conducted separation experiments of NPs by changing the packing material and the direction of the eluent supply. In addition, to confirm the properties of the NPs after purification using this method, we measured the modification state of the NPs.
Experiments
CeO₂ nanoparticles modified with decanoic acid, a fatty acid with a carbon chain length of 10, provided by the laboratory of Professor Adschiri at Tohoku University were used. The only pretreatment of NPs was the removal of unmodified fatty acid. The particle concentration of the NPs dispersion was set to 1 wt%. Cyclohexane was used as the dispersion and eluent solvent. A gel-type strongly basic anion exchange resin (DIAION SA11A) was used as a filler. The exchange group of the resin was prepared using the same decanoic acid as the modified fatty acid of NP. For comparison, glass beads that do not interact with NPs were also used as a filler. In each experiment, the column packed with a filler was vertically placed in an air thermostat chamber at 30°C. After injecting 1 cm³ of NPs dispersion from the column inlet, the eluent was supplied in an upward flow at a liner velocity of 1 cm/min. For comparison, another experiment in which the eluent flowed downward, was also conducted. In the analysis, the effluent from the column was collected at predetermined time intervals, and the particle size distribution (PSD) of each fraction was measured by dynamic light scattering (DLS). In addition, to confirm the modification state of the NPs after passing through the column, the binding state of the fatty acid on the surface was measured by Fourier transform infrared spectroscopy (FTIR).
Results and discussion
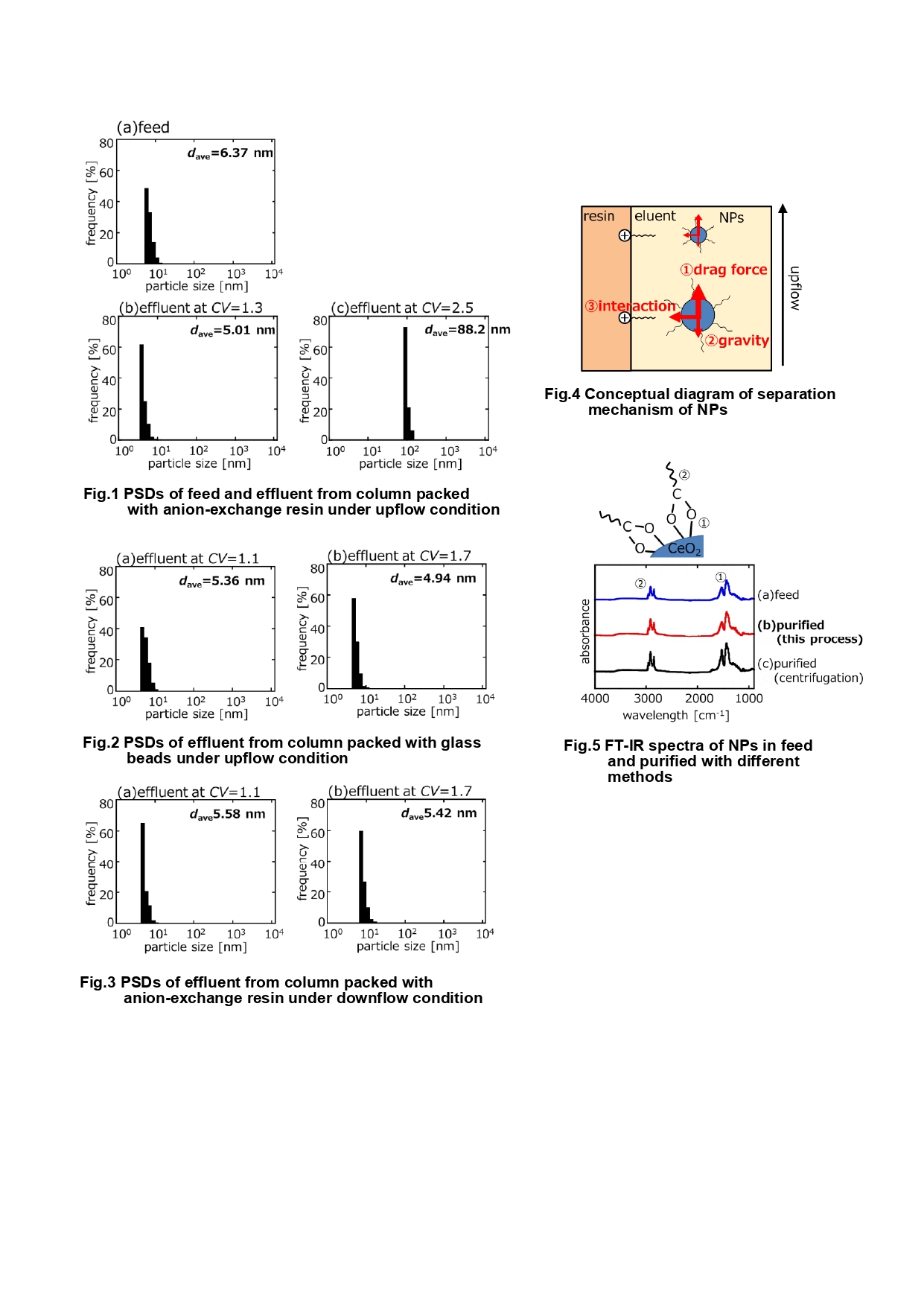
Figure 1 shows the particle size distributions (PSDs) of the feed dispersion and the effluent from the column packed with anion-exchange resin under upflow condition. CV is the elution volume normalized by the column void volume, and is an indicator of elution time. In CV = 1.3 (b), the PSD became narrower than in feed (a), and NPs with a smaller average particle diameter (dave) were obtained. On the other hand, in CV = 2.5 (b), NPS with a much larger dave were obtained. Therefore, this indicates that the separation of NPs by size was successful. Figure 2 shows the PSDs of the effluent from the column packed with glass beads under the upflow condition. At CV = 1.1, the PSD was similar to that of the feed dispersion. At CV = 1.7, the PSD was also similar to that of the feed dispersion, and no delayed elution of larger NPs was observed. These results indicate that the interaction between the resin and NPs is necessary for this purification. Figure 3 shows the PSDs of the effluent from the column packed with anion-exchange resin under the downflow condition. At CV = 1.1, the PSD became slightly narrower than that of the feed dispersion. Even at CV = 1.7, the PSD was also the same as that of the feed dispersion, and no delayed elution of larger NPs was observed. These results show that downflow is not sufficient to purify NPs. Therefore, the delayed elution of larger NPs in this purification is thought to be due not only to the interaction between the resin and NPs, but also to the effect of gravity on the flow of the effluent in the upstream direction. From the above, it can be concluded that the size separation in this purification involves: ①the drag force from the eluent flow, ②the gravity in the opposite direction to the drag force, and ③the interaction between the resin and NPs, as shown in Fig.4. Since all these forces become stronger for larger NPs, it is thought that the time for larger NPs to elute from the column is longer, leading to the size separation.
Figure 5 shows the FTIR spectra of the feed and the NPs purified using this method (Fig.1). For comparison, the FTIR spectra of the NPs purified by centrifugation is also shown. The spectra of the NPs purified using this method showed peaks (1440 cm-1, 1530 cm-1) due to the -COO- stretching vibration of the fatty acid bound to CeO2, and peaks (2850 cm-1, 2920 cm-1) due to the -CH2- stretching vibration of the fatty acid. The same peaks were observed in both the feed NPs and the NPs purified by centrifugation. Therefore, it is considered that this method allows purification without degrading its properties. This method is expected to be a much simpler NPs purification process than current methods, and it could lead to a breakthrough in practical applications.
References
1)M.Adschiri et al.,Adv.Mater.,19,203(2010)
2)T. Arita et al.,Nanoscale,2,1467(2010)
3 Hettiarachchi,S. et al.,Lab Chip,23,982–1010(2023)
4)T.Arita et al.,J.Nanopart.Res.,12,2567(2010)