2025 AIChE Annual Meeting
(458h) One-Step Conversion of Lignocellulosic Biomass to Bio-Based Amines
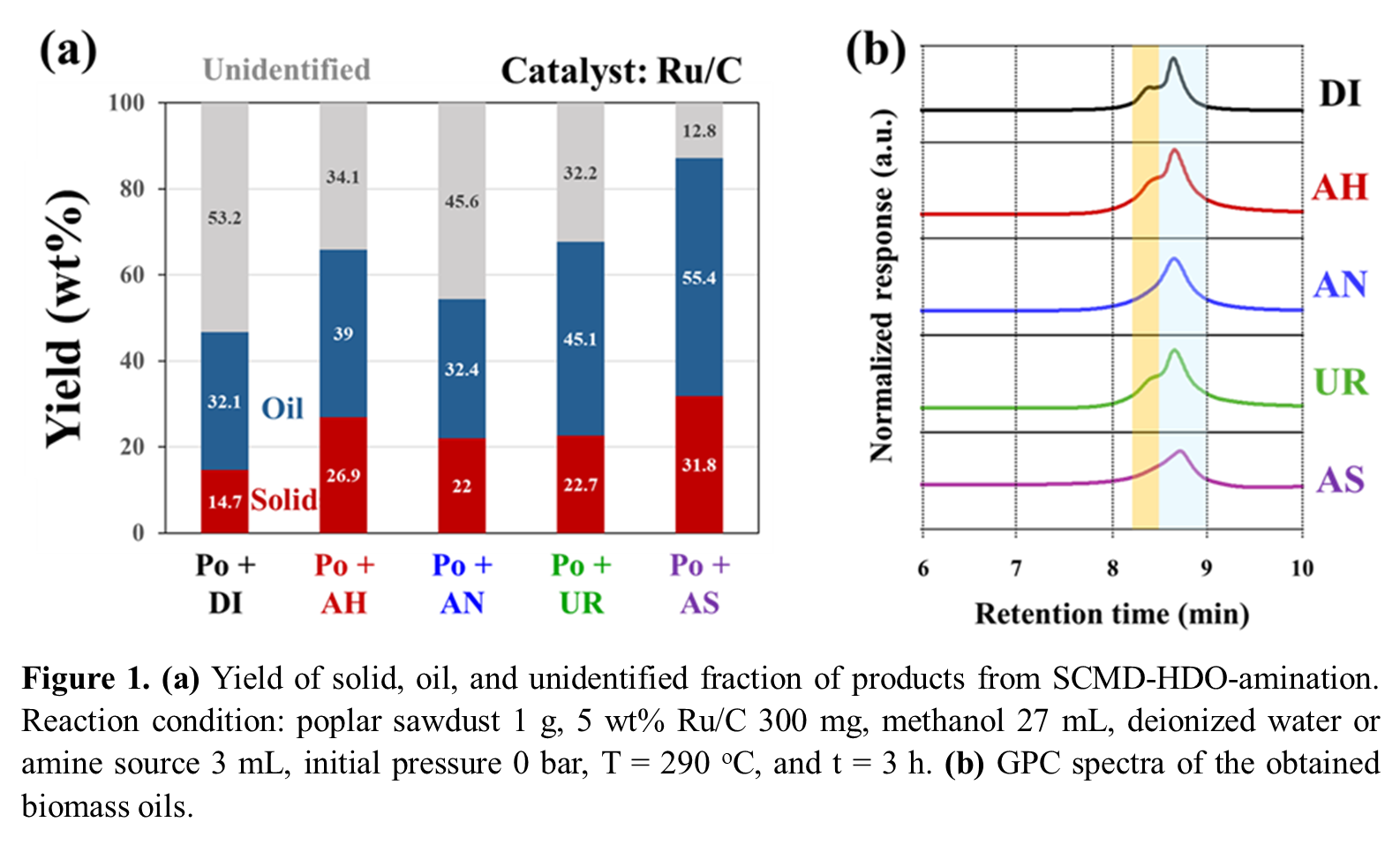
Inspired by this foundation, we present a one-step strategy for converting whole lignocellulosic biomass components into bio-based amines. Our approach integrates supercritical methanol depolymerization (SCMD), hydrodeoxygenation (HDO), and amination in a single reactor system. Using hybrid poplar (Po) as a feedstock, we evaluated the reaction with four nitrogen sources: ammonium hydroxide (AH), ammonium nitrate (AN), urea (UR), and ammonium sulfate (AS). Each compound served not only as N-donors for amination but also influenced SCMD efficiency. Our results demonstrate the significant impact of nitrogen sources on mass balance, oil yield, and product molecular weight distribution (Figure 1). To further investigate the underlying mechanism of this one-step reaction, we conducted the SCMD-HDO-amination with model compounds. Further details on reaction performance, process design, and optimization strategies will be presented at the upcoming AIChE meeting.