2025 AIChE Annual Meeting
(92d) From Offline to Inline: Advancing Inhalation Drug Manufacturing with Real-Time Particle Size Monitoring and Solids Concentration Modelling
Wet milling of Active Pharmaceutical Ingredients (API), via top-down micronization technologies such as Wet Polishing (WP) [1], enables precise particle sized reduction. Processing of API suspensions, in its antisolvent, is monitored and controlled by periodic in-process control (IPC) suspension samples that are analyzed by offline techniques.
Standard offline IPC by laser-diffraction technologies for particle size distribution (PSD), like Malvern’s Mastersizer, is often a time-consuming procedure, including sample collection, sample and dispersant preparation, as well as resources availability (manpower, equipment). Moreover, in cases in which PSD is a stop control, the turnaround time of offline measurements can impact not only cycle time but also increase the risk of process challenges (e.g.: sedimentation, agglomeration, clogging) and product loss. The loss of API to the equipment is responsible for the decrease of solids concentration in suspension, throughout time, which can potentially impact process performance when under inaccurate monitorization and control. Therefore, parallel and precise monitoring of PSD and solids concentration throughout the process is of utmost importance for process optimization.
Thus, the purpose of this work is two-fold:
- To assess the Single-Optical-Path Blaze technology, by Blaze Metrics, as alternative inline Process Analytical Technology (PAT) for PSD measurement.
- To develop inline and offline modelling for solids concentration in suspension, using Malvern obscuration and Blaze turbidity values against complementary particle size data. Thus, extending both techniques output by establishing a bidimensional monitorization tool to control product losses and process performance.
Ultimately, the goal is to corroborate and correlate Blaze performance with Malvern’s gold standard [2] and support the former’s implementation in suspension-based manufacturing processes and gradually reduce sampling and stop time.
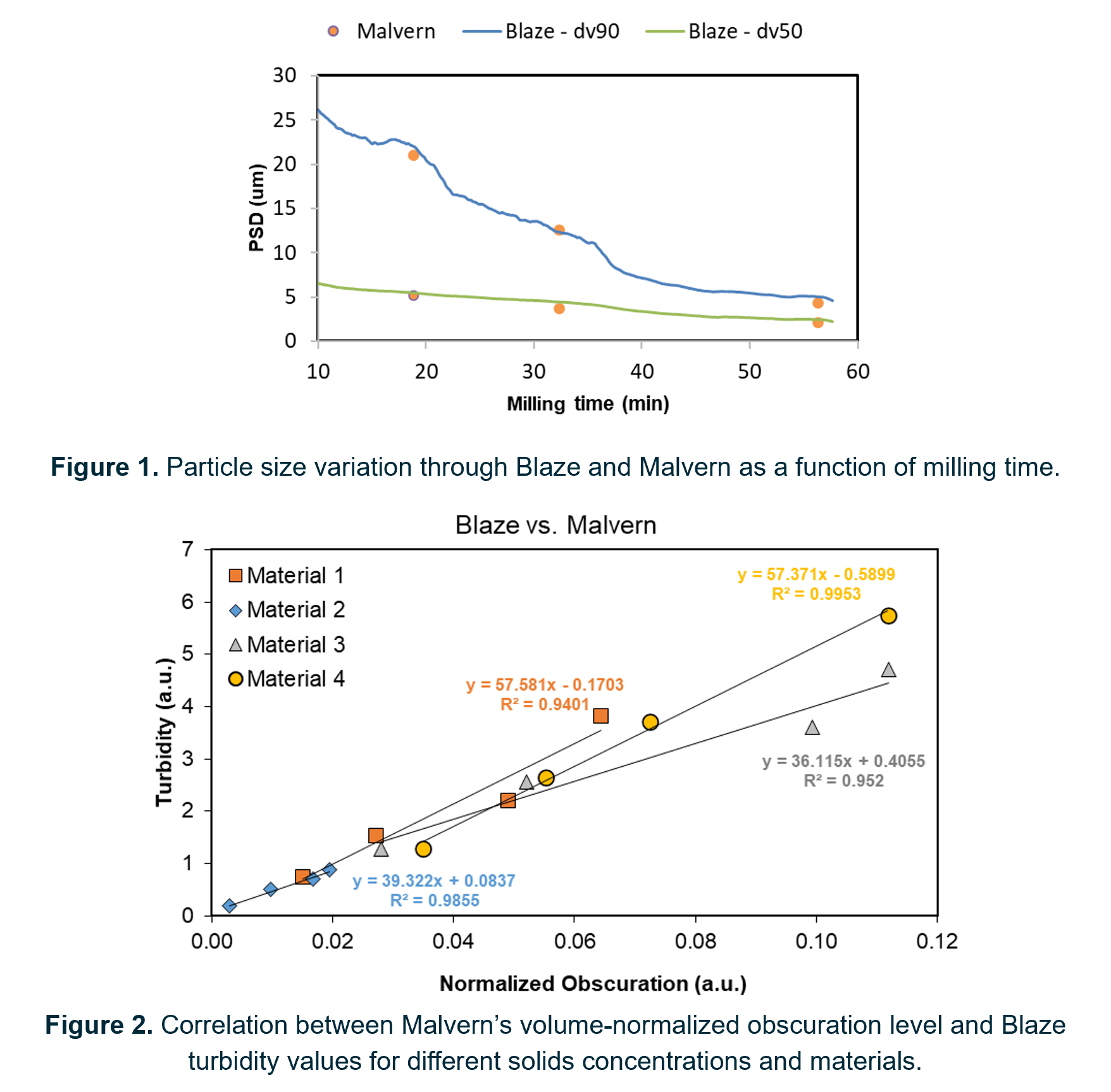
To assess the potential of Blaze to measure the particle size, wet milling was conducted using high shear mixer and high-pressure homogenizer equipment. Throughout the milling process, Blaze and Malvern performances were compared for different solid-antisolvent formulations. Initial data with homogeneous suspensions indicated high discrepancies between the PSD results of the two techniques, primarily affected by probe positioning and sampling location. By optimizing the probe’s inline setup, via flowcell, the impact on sample representativeness was significantly reduced. Additionally, considering Blaze’s dependency on imaging resolution, the use of lower Field of View (FOV) probes was tested. Subsequent monitorization during milling operations, with the improved setup, resulted in accurate and strongly correlated PSD determinations from the two techniques (R2 > 0.92) (Figure 1) even when tested for solids concentrations ranging between 2 – 12% w/w.
In parallel, to ascertain innovative methods to estimate solids in suspension, analytical protocols were optimized to fine tune Malvern’s obscuration data collection during particle size analysis and compared against the turbidity values recorded by Blaze for suspensions. The same solid-antisolvent formulations were prepared, at the same range of solids concentration, and tested. Consistent correlations were obtained between both technologies, as highlighted in Figure 2 (R2 > 0.94). The results show that Blaze measurements are directly related with volume-normalized Malvern obscuration and that both, combined with PSD results, can be used to robustly estimate solids concentration, regardless of the type of tested formulation.
Future work will focus on supporting the establishment of a standardized definition of suspension homogeneity, expanding testing to a broader range of formulations, and addressing the contribution of suspension-related challenges such as foaming, flocculation, and particle adhesion.
In conclusion, this study demonstrates the potential of Blaze technology as a real-time PAT tool for monitoring particle size distribution and solids concentration during the processing of inhalation pharmaceutical suspensions. Showing strong alignment with Malvern’s gold standard, Blaze enables continuous inline measurements while reducing contamination risk, process interruptions, and resource demands. This approach enhances process robustness, efficiency, and sustainability, supporting a gradual shift from traditional offline methods to more innovative and eco-friendly manufacturing practices.
References:
[1] WO2011131947A2 – A process for particle processing of active pharmaceutical ingredients.
[2] Shekunov et al (2007) Particle size analysis in pharmaceutics: principles, methods, and applications. Pharmaceutical Research 24 (2). DOI: 10.1007/s11095-006-9146-7