2025 AIChE Annual Meeting
(227k) Next-Generation Bio-Mimetic Scaffold for Long-Bone Regeneration: Harnessing Piezoelectric Self-Charging and Osteo-Inductive Cues
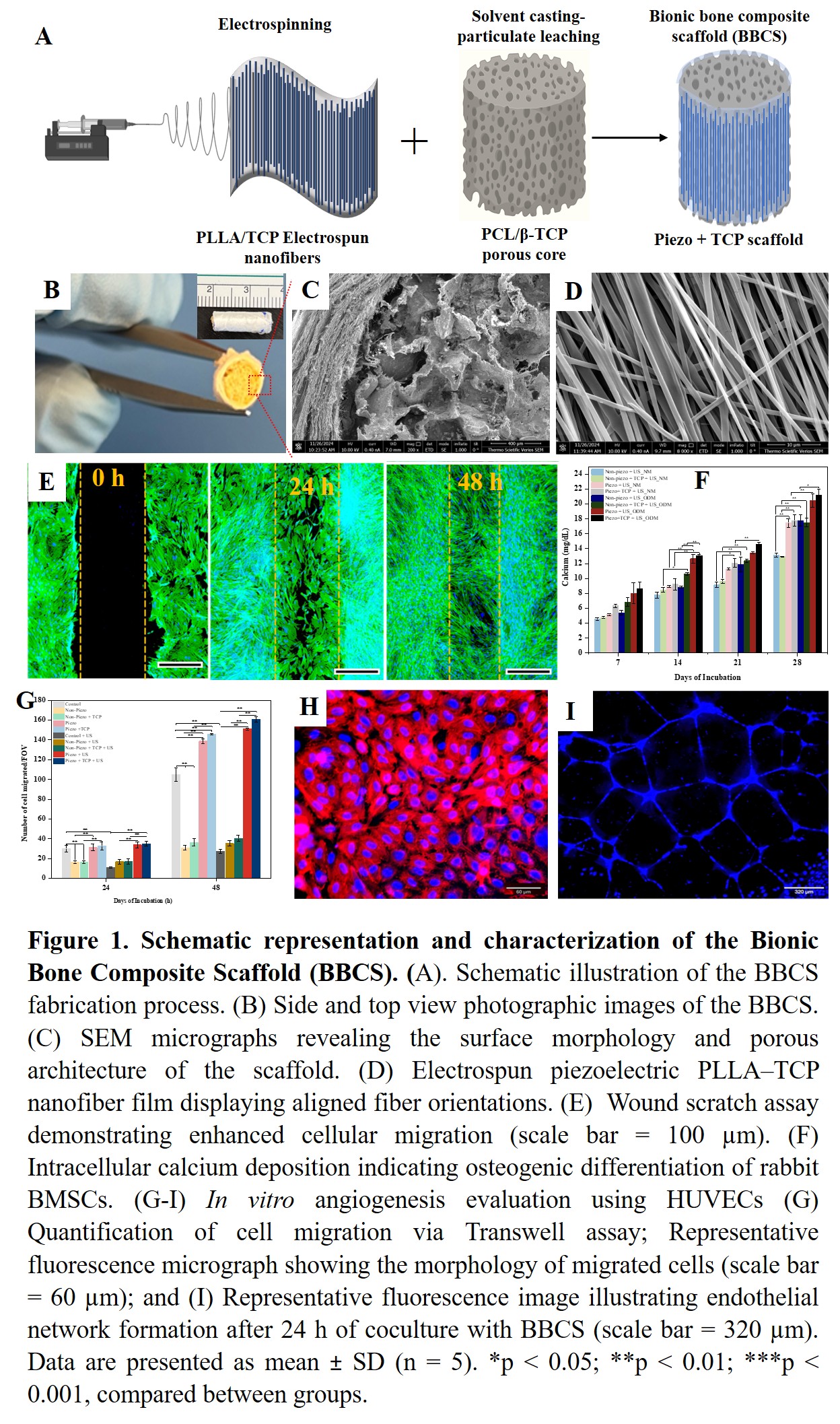
Here, we report the development of a biodegradable bionic bone composite scaffold (BBCS) with bone-mimetic architecture that can be remotely activated by ultrasound (US). This scaffold integrates the piezoelectric self-charging effect of PLLA/TCP electrospun nanofibers with the osteo-inductive ion release from β-tricalcium phosphate (β-TCP), yielding a synergistic mechanism not previously described. The BBCS was fabricated as a cylindrical long-bone construct by wrapping a piezoelectric PLLA/β-TCP nanofiber membrane around a porous polycaprolactone (PCL)/β-TCP core, produced via solvent casting and particle leaching. The electrospun composite nanofibers exhibited enhanced piezoelectric performance while retaining structural and biochemical features comparable to native bone. In vitro studies using rabbit bone marrow-derived mesenchymal stem cells (BMSCs) and human umbilical vein endothelial cells (HUVECs) demonstrated that ultrasound-activated BBCS significantly enhanced osteogenesis and angiogenesis. Specifically, piezoelectric stimulation accelerated vascular network formation in HUVECs and promoted robust osteogenic differentiation of BMSCs. The combined effects of piezoelectric charge, β-TCP ion release, and ultrasound activation facilitated cell recruitment, stimulated endogenous growth factor secretion, and augmented mineral deposition. We are now conducting an in vivo study to show bone healing in a critical-sized long-bone defect in New Zealand White Rabbits in order to validate our in vitro results.
Collectively, our findings validate the synergistic regenerative mechanism of the BBCS and underscore its potential as a next-generation bone scaffold. This strategy enables effective long-bone defect repair without the need for exogenous stem cells, growth factors, or pharmacological agents, offering a safe and translationally viable platform for clinical bone tissue engineering.