2025 AIChE Annual Meeting
(424g) A New Approach to Selective CO Removal from Hydrogen: Chemical Looping Preferential Oxidation and In-situ Immobilization
Authors
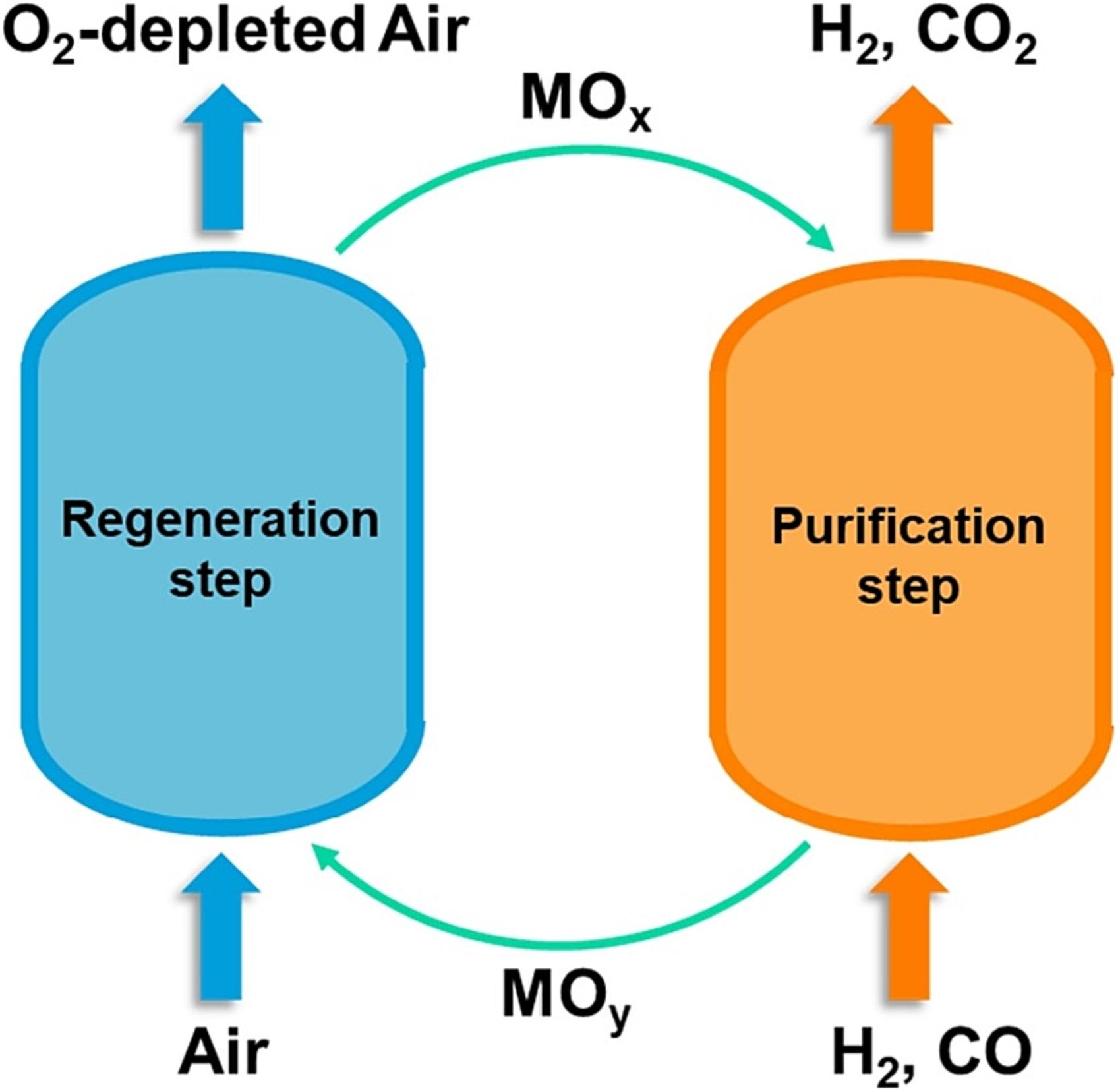
A novel chemical looping preferential oxidation (CL-PROX) process is proposed and demonstrated for eliminating trace CO from a H2-rich stream. CL-PROX is intrinsically safe and significantly alleviates the deactivation problem due to the superior reduction/reoxidation pattern. The proposed process exhibited an excellent CO removal performance (CO concentration < 100 ppm) and a high H2 recovery (>96 %) with a ceria-supported γ-Fe2O3 oxygen carrier. The structure and evolution of iron species in the oxygen carrier were investigated through experimental characterizations during activation and redox cycles. TPR characterizations illustrated a significant difference in the reducibility of the active species, γ-Fe2O3, under H2 and CO atmospheres, which could be the evidence for its relatively high selectivity to CO oxidation. In-situ FTIR characterization also pointed out that the desorption of reaction intermediates to CO2 is facilitated via the reduction of the surface γ-Fe2O3, which might contribute to the outstanding CO oxidation performance of the oxygen carrier.
The process is further intensified by dual function materials (DFM), enabling both catalytic CO preferential oxidation and in-situ immobilization. This enhancement leads to a near-complete CO conversion with a CO concentration below 20 ppm while achieving a hydrogen recovery of 97 % in the purification step. Characterizations indicate that CO can be converted into surface formate and carbonate on the DFM, which can readily decompose to CO2 during regeneration step.
In summary, this work introduces an intensified chemical looping strategy for hydrogen purification, addressing the stringent CO purity requirements for critical downstream applications such as fuel cells and ammonia synthesis. The integration of catalytic CO oxidation with in-situ immobilization through multifunctional redox materials not only enhances CO removal efficiency but also provides valuable insights into the rational design of advanced oxygen carriers and dual-function materials for clean energy processes.