2025 AIChE Annual Meeting
(400bb) Nanomaterial doped Polyethersulfone Hollow Fiber Membranes for Bioartificial Kidney and Hemodialysis Applications
To address this urgent healthcare gap, our research focuses on developing hollow fiber membranes based bioartificial organs system, the design and fabrication of next-generation polymeric hollow fiber membranes for biomedical applications, including hemodialysis, bioartificial kidney, and bioartificial liver systems. Our core innovation lies in engineering HFMs that not only provides highly efficient toxin removal including low molecular weight solutes (urea, creatinine), middle molecular weight proteins (e.g., lysozyme), and protein-bound uremic toxins (such as indoxyl sulfate) but also support cell adhesion, viability, and metabolic activity. The structural and functional properties of the HFMs were enhanced by employing various strategies such as blending of nanomaterials, their composite, polymer additive by direct blending during dope preparation and HFMs spinning along with coating of HFMs to provide the functional improvement.
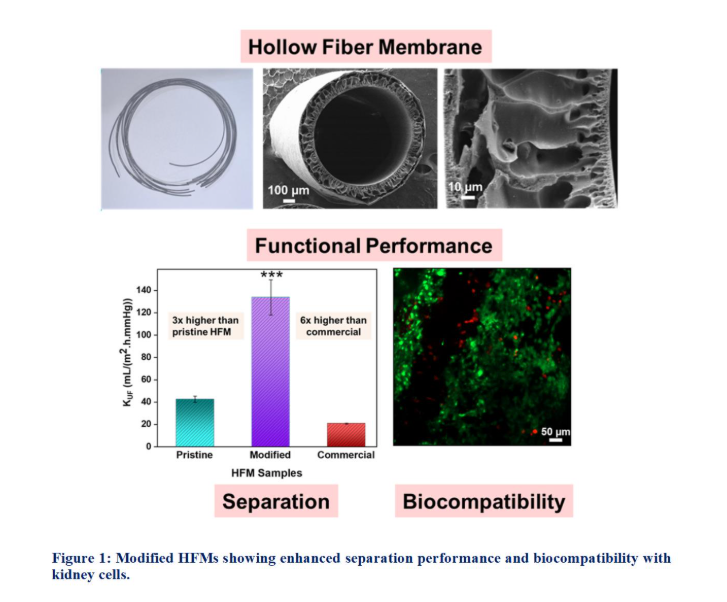
In this study, polyethersulfone (PES) hollow fiber membranes (HFMs) were modified by incorporating titanium dioxide (TiO2) and graphene oxide (GO) during the spinning process. This approach leverages the non-toxicity, hydrophilicity, and biocompatibility to enhance membrane properties for hemodialysis and bioartificial kidney applications. Comprehensive characterization and experimental analyses demonstrated that TiO2/GO PES-based HFMs exhibit superior suitability as a substrate for hemodialysis and bioartificial kidney (BAK) applications. Modified HFMs showed enhanced biocompatibility, supporting the adhesion, proliferation, and growth of HEK-293 cells. Confocal microscopy revealed extensive cell attachment, spreading, and spheroid formation on the TiO2/GO membranes. MTT assay confirmed a higher number of metabolically active viable cells, while flow cytometry-based live/dead assays indicated no cytotoxic effects on HEK293 cell lines. Hemocompatibility tests demonstrated non-hemolytic behaviour, confirming their safety for blood-contacting applications. Complement activation assays further indicated anti-inflammatory properties upon blood contact. Separation performance assessments revealed superior toxin removal efficiency for the TiO2/GO HFMs as compared to pristine HFMs, highlighting a balanced combination of cell proliferation support and toxin clearance. Based on these findings, TiO2/GO demonstrates superior biocompatibility, anti-inflammatory properties, and enhanced functional performance, making it a suitable candidate for utilization in hemodialysis and bioartificial kidney applications. [3], [4]
References:
[1] “OPTN/SRTR 2023 Annual Data Report”.
[2] “Notto India organ transplant data.”
[3] N. Pandey and J. Bellare, “Titanium dioxide/graphene oxide blending into polyethersulfone hollow fiber membranes improves biocompatibility and middle molecular weight separation for bioartificial kidney and hemodialysis applications,” J. Mater. Chem. B, p. 10.1039.D5TB00229J, 2025, doi: 10.1039/D5TB00229J.
[4] N. Pandey, N. Kar, and J. Bellare, “Titanium Dioxide (TiO2) Incorporation into Poly (ether sulfone) (PES) Hollow Fiber Membranes (HFMs) Improves Biocompatibility and Separation Performance for Bioartificial Kidney (BAK) and Hemodialysis Applications,” 2025, doi: 10.1021/acsabm.4c01309.