2025 AIChE Annual Meeting
(667h) Nanofluid -Toluene Biphasic Flow in Mesoscale - Reacting Vis-a-Vis Nonreacting Systems
Authors
- Introduction
Liquid-liquid immiscible flow plays a crucial role in various industrial applications, including extraction, emulsification, and reaction engineering. Several studies indicate that optimizing these flows significantly enhances mass transfer rate, thereby improving industrial processes. Effective mixing and interfacial area are key factors influencing mass transfer in liquid-liquid flow systems. Mass transfer in liquid-liquid flow reactions significantly affects conversion and selectivity and examples of this are chemical reactions like nitration, phase transfer catalysis, etc. Miniaturization instead of macro-scale operation has emerged as a promising approach for process intensification in heterogenous flow systems, as it enhances the range of plug (elongated droplets with length ≥ twice of conduit diameter) flow. This flow distribution increases the rate of transport processes due to higher interfacial area and internal circulation within the plugs, constantly refreshing the interface. Meso-channel flow quantified as 0.57 < Co < 4.46 (confinement number, Co=1/D √(σi /(gΔρ)), and Δρ, D, σi and g are difference in liquid densities, conduit diameter, interfacial tension and gravitational acceleration respectively) while exhibiting the advantages of reduced dimensions alleviates the disadvantages of microscale flow, namely high pressure drop, high tendency to clog etc. To further improve mass transfer performance, nanofluids (NFs) have been explored as a novel strategy [1]. The addition of nanoparticles in small concentrations (≤ 0.05 vol%) modifies the interfacial tension without significantly changing the density and viscosity of the base fluid. Our previous studies [2] have shown that NFs favors generation of uniform-sized plugs of organic phase over a wide range of biphasic flow conditions. In mesoscale flow reactors, uniform sized plugs are highly desirable while conducting multiphasic reactions. This ensures close to same processing experience of the plugs that serve as individual reactors. However, the reactant and products affect the flow characteristics of such systems. Here we explore a reacting flow system comprising of nanofluid (aqueous) and toluene (organic) and investigate the effect of reaction on mesoscale biphasic flow. Though the nanoparticles are mostly inert, their presence at the liquid-liquid interface may also affect the reactions. The reaction is performed with sodium hydroxide dissolved in the nanofluids and iodine in the organic phase. It may be noted that although a few studies have investigated the influence of phase distribution on conversion, the influence of reaction on phase distribution is relatively less explored.
- Experimental setup
The flow experiments are performed under both non-reacting and reacting conditions in a glass conduit 2.38mm diameter (D) and 1m long. For non-reacting experiments, the test fluids are toluene dyed red with iodine (0.1 gm/500 mL) as the organic phase and deionized water (W) and nanofluid (NF) as the aqueous phase. The nanofluid comprises of 0.01 vol% Al2O3 nanoparticles and 0.2 wt% sodium dodecyl sulfate (SDS) (relative to the nanoparticle mass) to stabilize the nanofluid. Prior to experimentation for the non-reacting cases, the immiscible phases are mixed and allowed to stand overnight in a tank, ensuring mutual immiscibility during flow [2]. For reacting systems, 0.5 M sodium hydroxide (NaOH) is added to the aqueous phase, and it reacts with 0.01 M iodine of the organic phase during flow as per the following equation.
3I2(org)+6NaOH(aq)→5NaI(aq)+NaIO3(aq)+3H2O.
The reactants are highly soluble in their respective phases and the products exhibit high solubility in the aqueous phase but are insoluble in the organic phase [3]. Due to excess NaOH, iodine acts as the limiting reactant for most of the flow conditions and the reaction can be considered as pseudo first order [3]. The physical properties of the test liquids are listed in Table 1. The two liquids are introduced into the 1 m long conduit through opposite ends of a T-entry for up flow, using a dual syringe pump, with flow rates ranging from 2 to 80 mL/min. A high-speed camera, positioned at 50 cm from the inlet records the phase distribution for the different flow rate combinations in the fully developed flow region and a second camera is focused at the inlet to observe the droplet pinch-off mechanism. Additional experiments are performed using surfactant solution (S) as the aqueous phase with and without reaction to delineate the influence of surfactant and nanoparticle-surfactant assembly (NPS) on the phase distribution.
- Results and Discussions
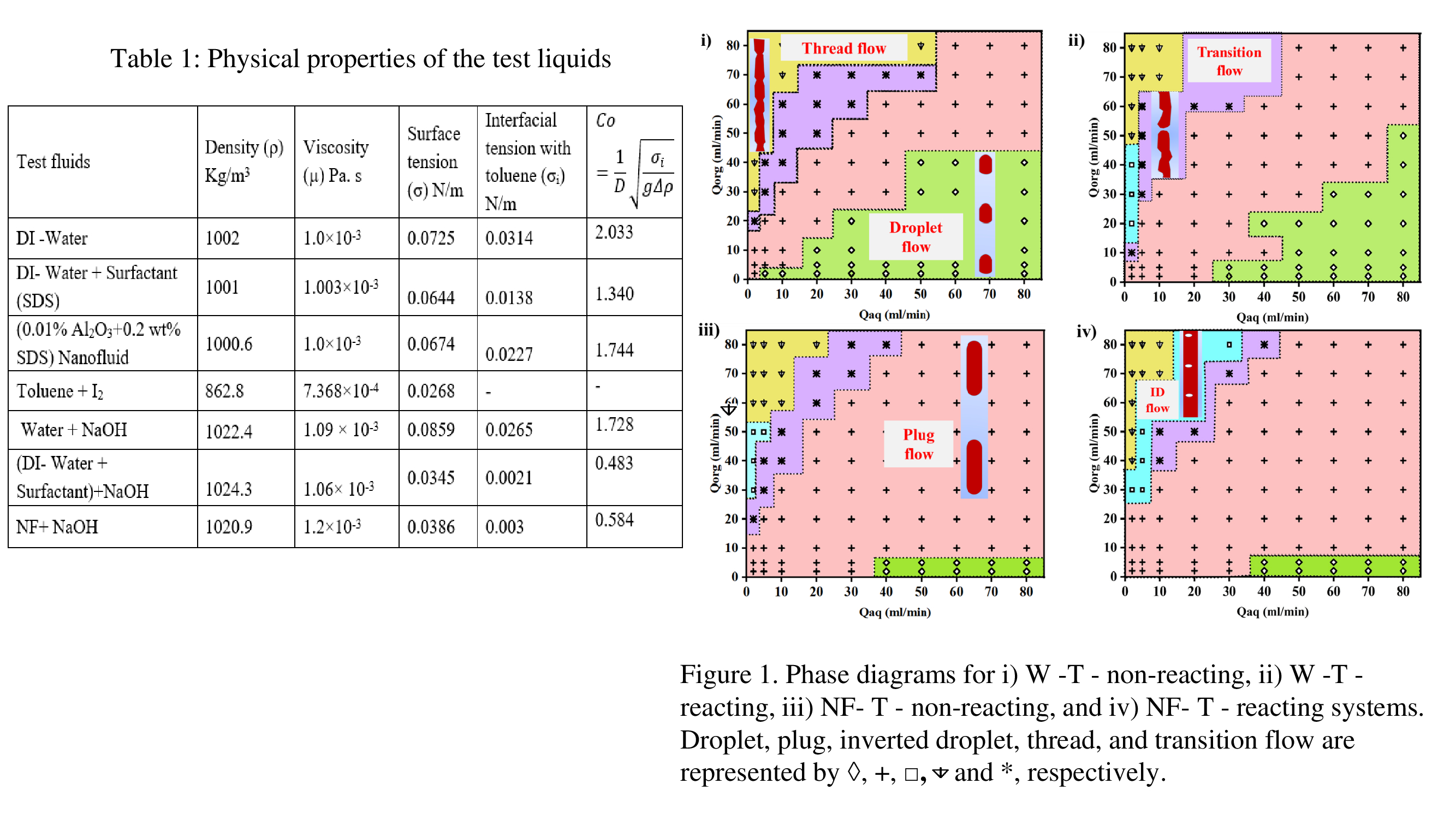
Figure 1 presents the range of existence of the observed flow patterns under reacting and non-reacting conditions for water (W)-toluene (T) and nanofluid (NF)-toluene (T) flow. Representative photographs of the phase distribution are inserted in the phase diagram. Droplet/ plug flow occurs when the organic phase disperses in the aqueous phase. As is evident from the figure, plug flow is characterised by droplet length (Ld) ≥ 2D and a continuous thread of toluene is noted within an aqueous phase annulus film (thread flow) at high organic and low aqueous flow. The transition between the two is characterized by a random distribution of organic plugs and threads. Under certain conditions of low aqueous and moderate organic flow, aqueous phase disperses as droplets in the organic phase (inverted droplet flow).
A comparative study of the 4 figures reveal –
- In non-reacting systems (Figures 1(i) and (iii)), nanofluids significantly enhance plug flow. This can be attributed to a lower interfacial tension (Table 1) which decreases capillary pressure and delays pinch-off, leading to plug formation. Furthermore, unlike water-toluene systems, nanofluids facilitate the formation of inverted droplets in lieu of thread flow under moderate organic flow rates (15 mL/min ≤ Qorg≤ 45 mL/min).
- Inverted droplet flow (ID) is noted for water-toluene flow under reacting conditions. This is possibly because toluene flow towards the conduit wall is assisted by the outer diffusion of iodine from the organic phase to the aqueous phase. The range increases towards higher flow rates of NF-T flow, due to increased propensity of NPS assemblies to create additional interface and facilitate interface jamming.
- The range of plug and transition flow expands, while the range of droplet flow decreases slightly in reacting systems.
- Observations at the entry shows that the droplet formation mechanisms are also significantly influenced by reaction and the range of jetting is higher in the presence of reaction and with nanofluids compared to water (not shown in the figure).
- Additionally, the range of monodispersity is also higher for reacting cases for NF-T flow while it is lower for reacting cases in case of W-T flow.
- Conclusion
This study offers insights into the influence of nanofluids on phase distribution in reacting as well as non-reacting biphasic liquid flow. Compared to non-reacting systems, under reaction (i) plug flow range is increased, (ii) inverted droplet flow is favored and (iii) transition flow gets reduced. This suggests that a straightforward extension of the flow patterns in non-reacting systems cannot be done for reacting cases and fresh experiments are required to investigate flow morphology under reacting conditions for different aqueous and organic phases selected. The altered flow patterns is possibly the combined effect of the presence of nanoparticles and surfactant at the interface and the reaction taking place at the interface.
- References
[1] Seyedeh-Saba Ashrafmansouri, Mohsen Nasr Esfahany, “Mass transfer in nanofluids: A review,” International Journal of Thermal Sciences, Volume 82, (2014), https://doi.org/10.1016/j.ijthermalsci.2014.03.017.
[2] Kunderu Pallavi, Alex Koshy, Gargi Das, Chirodeep Bakli, Subhabrata Ray, Nanofluid induced continuous production of monodispersed plugs during biphasic liquid flow in meso-scale, Chemical Engineering and Processing - Process Intensification, Volume 209, 2025, https://doi.org/10.1016/j.cep.2025.110193.
[3] A. Koshy, B. Samanta, S. Ray, and G. Das, “The interplay of reaction and flow hydrodynamics in multiphase millireactor,” Journal of Industrial and Engineering Chemistry, Volume 113, (2022), https://doi.org/10.1016/j.jiec.2022.06.032.