2025 AIChE Annual Meeting
(626b) Nanofilm Composite Membranes with Mixed Matrix Materials Achieving Superior CO2/N2 Separation Performance
Authors
Narjes Esmaeili - Presenter, University at Buffalo, The State University of New York
Shiwen Dong, University at Buffalo, The State University of New York
Vinh Bui, University at Buffalo
Haiqing Lin, University of Buffalo, State University of New Yor
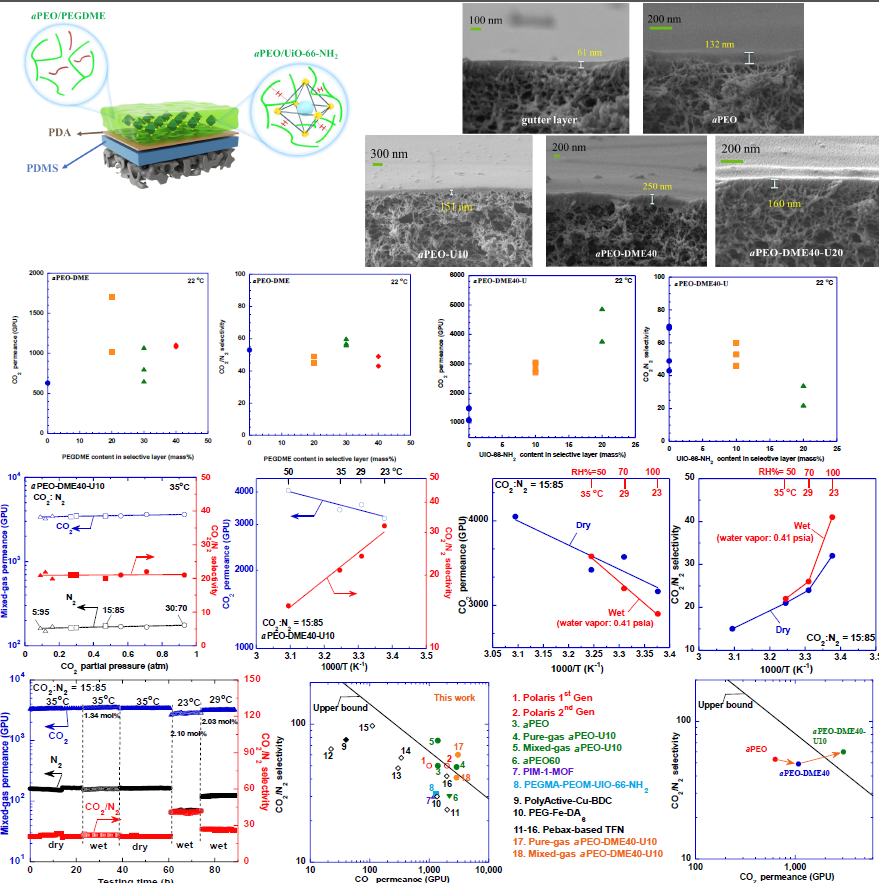
Mixed matrix materials (MMMs) integrating excellent processability from polymers and distinct separation properties from metal-organic frameworks (MOFs) are of interest for membrane gas separations, and they are often made into freestanding films (> 100 µm) to demonstrate superior gas separation properties. However, they are difficult to fabricate into nanofilm composite membranes due to interfacial incompatibility between polymers and nanofillers. Here we have successfully developed TFN membranes based on MMMs (as thin as 100 nm) comprising amorphous poly(ethylene oxide) (aPEO) and UiO-66-NH2 (45-85 nm) enabled by strong hydrogen bonds between the two matrices. A third component of liquid polyethylene glycol dimethyl ether (PEGDME, 240 Da) can be incorporated in the aPEO to improve CO2 permeance while retaining CO2/N2 selectivity. For example, adding 10 wt% UiO-66-NH2 in aPEO increases CO2 permeance from 630 to 1700 GPU while increasing CO2/N2 selectivity to 62; incorporating 40 wt% PEGDME into the selective layer dramatically increases CO2 permeance from 1700 to 3000 GPU while maintaining CO2/N2 selectivity at 60, surpassing Robeson’s upper bound and commercial membrane such as Polaris 2nd Gen (with pure-gas CO2 permeance of 1700 GPU and CO2/N2 selectivity of 50). An example membrane (containing 40% PEGDME and 10% UiO66-NH2) demonstrates mixed-gas CO2 permeance of 2900 GPU and CO2/N2 selectivity of 41 in the presence of 2.1 mol% water vapor at ≈23 °C. The relationships between the membrane nanostructures and CO2 separation properties will be elucidated.